Angiotensin AT1 and AT2 receptor heteromer expression in the hemilesioned rat model of Parkinson's disease that increases with levodopa-induced dyskinesia
- PMID: 32807174
- PMCID: PMC7430099
- DOI: 10.1186/s12974-020-01908-z
Angiotensin AT1 and AT2 receptor heteromer expression in the hemilesioned rat model of Parkinson's disease that increases with levodopa-induced dyskinesia
Abstract
Background/aims: The renin-angiotensin system (RAS) is altered in Parkinson's disease (PD), a disease due to substantia nigra neurodegeneration and whose dopamine-replacement therapy, using the precursor levodopa, leads to dyskinesias as the main side effect. Angiotensin AT1 and AT2 receptors, mainly known for their role in regulating water homeostasis and blood pressure and able to form heterodimers (AT1/2Hets), are present in the central nervous system. We assessed the functionality and expression of AT1/2Hets in Parkinson disease (PD).
Methods: Immunocytochemistry was used to analyze the colocalization between angiotensin receptors; bioluminescence resonance energy transfer was used to detect AT1/2Hets. Calcium and cAMP determination, MAPK activation, and label-free assays were performed to characterize signaling in homologous and heterologous systems. Proximity ligation assays were used to quantify receptor expression in mouse primary cultures and in rat striatal sections.
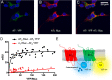
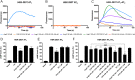
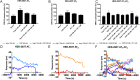
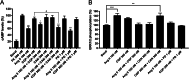
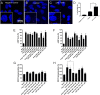
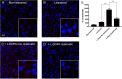
Results: We confirmed that AT1 and AT2 receptors form AT1/2Hets that are expressed in cells of the central nervous system. AT1/2Hets are novel functional units with particular signaling properties. Importantly, the coactivation of the two receptors in the heteromer reduces the signaling output of angiotensin. Remarkably, AT1/2Hets that are expressed in both striatal neurons and microglia make possible that candesartan, the antagonist of AT1, increases the effect of AT2 receptor agonists. In addition, the level of striatal expression increased in the unilateral 6-OH-dopamine lesioned rat PD model and was markedly higher in parkinsonian-like animals that did not become dyskinetic upon levodopa chronic administration if compared with expression in those that became dyskinetic.
Conclusion: The results indicate that boosting the action of neuroprotective AT2 receptors using an AT1 receptor antagonist constitutes a promising therapeutic strategy in PD.
Keywords: Dyskinesia; G-protein-coupled receptor (GPCR); Heteromer; Levodopa; Neuroinflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Novel Interactions Involving the Mas Receptor Show Potential of the Renin-Angiotensin system in the Regulation of Microglia Activation: Altered Expression in Parkinsonism and Dyskinesia.Neurotherapeutics. 2021 Apr;18(2):998-1016. doi: 10.1007/s13311-020-00986-4. Epub 2021 Jan 20. Neurotherapeutics. 2021. PMID: 33474655 Free PMC article.
-
Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: implications for progression of Parkinson's disease.Eur J Neurosci. 2010 Nov;32(10):1695-706. doi: 10.1111/j.1460-9568.2010.07448.x. Epub 2010 Oct 21. Eur J Neurosci. 2010. PMID: 20964730
-
The cannabinoid CB1 receptor interacts with the angiotensin AT2 receptor. Overexpression of AT2-CB1 receptor heteromers in the striatum of 6-hydroxydopamine hemilesioned rats.Exp Neurol. 2023 Apr;362:114319. doi: 10.1016/j.expneurol.2023.114319. Epub 2023 Jan 9. Exp Neurol. 2023. PMID: 36632949
-
The role of the brain renin-angiotensin system in Parkinson´s disease.Transl Neurodegener. 2024 Apr 15;13(1):22. doi: 10.1186/s40035-024-00410-3. Transl Neurodegener. 2024. PMID: 38622720 Free PMC article. Review.
-
The AT1/AT2 Receptor Equilibrium Is a Cornerstone of the Regulation of the Renin Angiotensin System beyond the Cardiovascular System.Molecules. 2023 Jul 18;28(14):5481. doi: 10.3390/molecules28145481. Molecules. 2023. PMID: 37513355 Free PMC article. Review.
Cited by
-
Pathogenesis of Chronic Kidney Disease Is Closely Bound up with Alzheimer's Disease, Especially via the Renin-Angiotensin System.J Clin Med. 2023 Feb 12;12(4):1459. doi: 10.3390/jcm12041459. J Clin Med. 2023. PMID: 36835994 Free PMC article. Review.
-
Potential protective role of ACE-inhibitors and AT1 receptor blockers against levodopa-induced dyskinesias: a retrospective case-control study.Neural Regen Res. 2021 Dec;16(12):2475-2478. doi: 10.4103/1673-5374.313061. Neural Regen Res. 2021. PMID: 33907036 Free PMC article.
-
Profiling novel pharmacology of receptor complexes using Receptor-HIT.Biochem Soc Trans. 2021 Aug 27;49(4):1555-1565. doi: 10.1042/BST20201110. Biochem Soc Trans. 2021. PMID: 34436548 Free PMC article. Review.
-
Cannabinoid regulation of angiotensin II-induced calcium signaling in striatal neurons.NPJ Parkinsons Dis. 2024 Nov 15;10(1):220. doi: 10.1038/s41531-024-00827-7. NPJ Parkinsons Dis. 2024. PMID: 39548112 Free PMC article.
-
Neuroprotection afforded by targeting G protein-coupled receptors in heteromers and by heteromer-selective drugs.Front Pharmacol. 2023 Jul 13;14:1222158. doi: 10.3389/fphar.2023.1222158. eCollection 2023. Front Pharmacol. 2023. PMID: 37521478 Free PMC article. Review.
References
-
- Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

