Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles
- PMID: 32796745
- PMCID: PMC7465149
- DOI: 10.3390/microorganisms8081222
Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles
Abstract
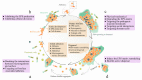
Biofilms are aggregate of microorganisms in which cells are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS) and adhere to each other and/or to a surface. The development of biofilm affords pathogens significantly increased tolerances to antibiotics and antimicrobials. Up to 80% of human bacterial infections are biofilm-associated. Dispersal of biofilms can turn microbial cells into their more vulnerable planktonic phenotype and improve the therapeutic effect of antimicrobials. In this review, we focus on multiple therapeutic strategies that are currently being developed to target important structural and functional characteristics and drug resistance mechanisms of biofilms. We thoroughly discuss the current biofilm targeting strategies from four major aspects-targeting EPS, dispersal molecules, targeting quorum sensing, and targeting dormant cells. We explain each aspect with examples and discuss the main hurdles in the development of biofilm dispersal agents in order to provide a rationale for multi-targeted therapy strategies that target the complicated biofilms. Biofilm dispersal is a promising research direction to treat biofilm-associated infections in the future, and more in vivo experiments should be performed to ensure the efficacy of these therapeutic agents before being used in clinic.
Keywords: AMP; EPS; antibodies; biofilm; enzyme; metabolic; microbial resistance; quorum sensing.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Figures
Similar articles
-
Therapeutic Strategies against Biofilm Infections.Life (Basel). 2023 Jan 6;13(1):172. doi: 10.3390/life13010172. Life (Basel). 2023. PMID: 36676121 Free PMC article. Review.
-
Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal.Folia Microbiol (Praha). 2018 Jul;63(4):413-432. doi: 10.1007/s12223-018-0585-4. Epub 2018 Jan 19. Folia Microbiol (Praha). 2018. PMID: 29352409 Review.
-
Approaches to Dispersing Medical Biofilms.Microorganisms. 2017 Apr 1;5(2):15. doi: 10.3390/microorganisms5020015. Microorganisms. 2017. PMID: 28368320 Free PMC article. Review.
-
Understanding the intricacies of microbial biofilm formation and its endurance in chronic infections: a key to advancing biofilm-targeted therapeutic strategies.Arch Microbiol. 2024 Feb 1;206(2):85. doi: 10.1007/s00203-023-03802-7. Arch Microbiol. 2024. PMID: 38300317 Review.
-
Strategy to combat biofilms: a focus on biofilm dispersal enzymes.NPJ Biofilms Microbiomes. 2023 Sep 7;9(1):63. doi: 10.1038/s41522-023-00427-y. NPJ Biofilms Microbiomes. 2023. PMID: 37679355 Free PMC article. Review.
Cited by
-
Polysaccharides' Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens.Int J Mol Sci. 2023 Feb 17;24(4):4030. doi: 10.3390/ijms24044030. Int J Mol Sci. 2023. PMID: 36835442 Free PMC article. Review.
-
Insights on Pseudomonas aeruginosa Carbohydrate Binding from Profiles of Cystic Fibrosis Isolates Using Multivalent Fluorescent Glycopolymers Bearing Pendant Monosaccharides.Microorganisms. 2024 Apr 16;12(4):801. doi: 10.3390/microorganisms12040801. Microorganisms. 2024. PMID: 38674745 Free PMC article.
-
Shifting from Ammonium to Phosphonium Salts: A Promising Strategy to Develop Next-Generation Weapons against Biofilms.Pharmaceutics. 2024 Jan 5;16(1):80. doi: 10.3390/pharmaceutics16010080. Pharmaceutics. 2024. PMID: 38258091 Free PMC article. Review.
-
Effect of Spermidine on Biofilm Formation in Escherichia coli K-12.J Bacteriol. 2021 Apr 21;203(10):e00652-20. doi: 10.1128/JB.00652-20. Print 2021 Apr 21. J Bacteriol. 2021. PMID: 33685971 Free PMC article.
-
The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin.Molecules. 2021 Oct 22;26(21):6392. doi: 10.3390/molecules26216392. Molecules. 2021. PMID: 34770799 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

