Epithelial-Mesenchymal Transition in Asthma Airway Remodeling Is Regulated by the IL-33/CD146 Axis
- PMID: 32793232
- PMCID: PMC7387705
- DOI: 10.3389/fimmu.2020.01598
Epithelial-Mesenchymal Transition in Asthma Airway Remodeling Is Regulated by the IL-33/CD146 Axis
Abstract
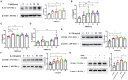
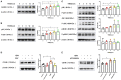
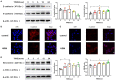
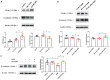
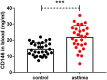
Epithelial-mesenchymal transition (EMT) is essential in asthma airway remodeling. IL-33 from epithelial cells is involved in pulmonary fibrosis. CD146 has been extensively explored in cancer-associated EMT. Whether IL-33 regulates CD146 in the EMT process associated with asthma airway remodeling is still largely unknown. We hypothesized that EMT in airway remodeling was regulated by the IL-33/CD146 axis. House dust mite (HDM) extract increased the expression of IL-33 and CD146 in epithelial cells. Increased expression of CD146 in HDM-treated epithelial cells could be blocked with an ST2-neutralizing antibody. Moreover, HDM-induced EMT was dependent on the CD146 and TGF-β/SMAD-3 signaling pathways. IL-33 deficiency decreased CD146 expression and alleviated asthma severity. Similarly, CD146 deficiency mitigated EMT and airway remodeling in a murine model of chronic allergic airway inflammation. Furthermore, CD146 expression was significantly elevated in asthma patients. We concluded that IL-33 from HDM extract-treated alveolar epithelial cells stimulated CD146 expression, promoting EMT in airway remodeling in chronic allergic inflammation.
Keywords: CD146; IL-33; allergy; asthma; epithelial-mesenchymal transition.
Copyright © 2020 Sun, Ji, Ma, Zhu, Chen, Wang, Qian, Wu, Hu, Huang and Zhang.
Figures


Similar articles
-
The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma.Clin Exp Allergy. 2016 Mar;46(3):479-90. doi: 10.1111/cea.12683. Clin Exp Allergy. 2016. PMID: 26609909
-
IL-37 protects against airway remodeling by reversing bronchial epithelial-mesenchymal transition via IL-24 signaling pathway in chronic asthma.Respir Res. 2022 Sep 13;23(1):244. doi: 10.1186/s12931-022-02167-7. Respir Res. 2022. PMID: 36100847 Free PMC article.
-
Huangqi-Fangfeng protects against allergic airway remodeling through inhibiting epithelial-mesenchymal transition process in mice via regulating epithelial derived TGF-β1.Phytomedicine. 2019 Nov;64:153076. doi: 10.1016/j.phymed.2019.153076. Epub 2019 Aug 23. Phytomedicine. 2019. PMID: 31473579
-
Research Advances in the Role of Bromodomain-containing Protein 4 in Epithelial-mesenchymal Transition in Asthma.Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017 Jun 20;39(3):425-431. doi: 10.3881/j.issn.1000-503X.2017.03.022. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017. PMID: 28695816 Review.
-
Approach for Elucidating the Molecular Mechanism of Epithelial to Mesenchymal Transition in Fibrosis of Asthmatic Airway Remodeling Focusing on Cl- Channels.Int J Mol Sci. 2023 Dec 25;25(1):289. doi: 10.3390/ijms25010289. Int J Mol Sci. 2023. PMID: 38203460 Free PMC article. Review.
Cited by
-
LincR-PPP2R5C Deficiency Alleviates Airway Remodeling by Inhibiting Epithelial-Mesenchymal Transition Through the PP2A/TGF-β1 Signaling Pathway in Chronic Experimental Allergic Asthma.Allergy Asthma Immunol Res. 2024 Jul;16(4):422-433. doi: 10.4168/aair.2024.16.4.422. Allergy Asthma Immunol Res. 2024. PMID: 39155740 Free PMC article.
-
Biomaterial-based mechanical regulation facilitates scarless wound healing with functional skin appendage regeneration.Mil Med Res. 2024 Feb 18;11(1):13. doi: 10.1186/s40779-024-00519-6. Mil Med Res. 2024. PMID: 38369464 Free PMC article. Review.
-
Wnt5a-mediated autophagy contributes to the epithelial-mesenchymal transition of human bronchial epithelial cells during asthma.Mol Med. 2024 Jun 19;30(1):93. doi: 10.1186/s10020-024-00862-3. Mol Med. 2024. PMID: 38898476 Free PMC article.
-
A Review on Asthma and Allergy: Current Understanding on Molecular Perspectives.J Clin Med. 2024 Sep 27;13(19):5775. doi: 10.3390/jcm13195775. J Clin Med. 2024. PMID: 39407835 Free PMC article. Review.
-
Extracellular Vesicles (EVs) as Crucial Mediators of Cell-Cell Interaction in Asthma.Int J Mol Sci. 2023 Feb 28;24(5):4645. doi: 10.3390/ijms24054645. Int J Mol Sci. 2023. PMID: 36902079 Free PMC article. Review.
References
-
- Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, et al. . Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. (2009) 180:122–33. 10.1164/rccm.200811-1730OC - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

