Prominent microglial inclusions in transgenic mouse models of α-synucleinopathy that are distinct from neuronal lesions
- PMID: 32787922
- PMCID: PMC7425556
- DOI: 10.1186/s40478-020-00993-8
Prominent microglial inclusions in transgenic mouse models of α-synucleinopathy that are distinct from neuronal lesions
Abstract
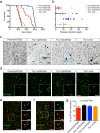
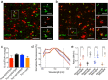
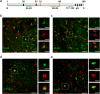
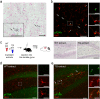
Alpha-synucleinopathies are a group of progressive neurodegenerative disorders, characterized by intracellular deposits of aggregated α-synuclein (αS). The clinical heterogeneity of these diseases is thought to be attributed to conformers (or strains) of αS but the contribution of inclusions in various cell types is unclear. The aim of the present work was to study αS conformers among different transgenic (TG) mouse models of α-synucleinopathies. To this end, four different TG mouse models were studied (Prnp-h[A53T]αS; Thy1-h[A53T]αS; Thy1-h[A30P]αS; Thy1-mαS) that overexpress human or murine αS and differed in their age-of-symptom onset and subsequent disease progression. Postmortem analysis of end-stage brains revealed robust neuronal αS pathology as evidenced by accumulation of αS serine 129 (p-αS) phosphorylation in the brainstem of all four TG mouse lines. Overall appearance of the pathology was similar and only modest differences were observed among additionally affected brain regions. To study αS conformers in these mice, we used pentameric formyl thiophene acetic acid (pFTAA), a fluorescent dye with amyloid conformation-dependent spectral properties. Unexpectedly, besides the neuronal αS pathology, we also found abundant pFTAA-positive inclusions in microglia of all four TG mouse lines. These microglial inclusions were also positive for Thioflavin S and showed immunoreactivity with antibodies recognizing the N-terminus of αS, but were largely p-αS-negative. In all four lines, spectral pFTAA analysis revealed conformational differences between microglia and neuronal inclusions but not among the different mouse models. Concomitant with neuronal lesions, microglial inclusions were already present at presymptomatic stages and could also be induced by seeded αS aggregation. Although nature and significance of microglial inclusions for human α-synucleinopathies remain to be clarified, the previously overlooked abundance of microglial inclusions in TG mouse models of α-synucleinopathy bears importance for mechanistic and preclinical-translational studies.
Keywords: Amyloid; Conformation; Inclusion; Microglia; Parkinson’s disease; Prion-like; Synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Microglial inclusions and neurofilament light chain release follow neuronal α-synuclein lesions in long-term brain slice cultures.Mol Neurodegener. 2021 Aug 11;16(1):54. doi: 10.1186/s13024-021-00471-2. Mol Neurodegener. 2021. PMID: 34380535 Free PMC article.
-
Comparative analyses of the in vivo induction and transmission of α-synuclein pathology in transgenic mice by MSA brain lysate and recombinant α-synuclein fibrils.Acta Neuropathol Commun. 2019 May 20;7(1):80. doi: 10.1186/s40478-019-0733-3. Acta Neuropathol Commun. 2019. PMID: 31109378 Free PMC article.
-
Toxic properties of microsome-associated alpha-synuclein species in mouse primary neurons.Neurobiol Dis. 2018 Mar;111:36-47. doi: 10.1016/j.nbd.2017.12.004. Epub 2017 Dec 12. Neurobiol Dis. 2018. PMID: 29246724 Free PMC article.
-
Dissecting α-synuclein inclusion pathology diversity in multiple system atrophy: implications for the prion-like transmission hypothesis.Lab Invest. 2019 Jul;99(7):982-992. doi: 10.1038/s41374-019-0198-9. Epub 2019 Feb 8. Lab Invest. 2019. PMID: 30737468 Free PMC article. Review.
-
Vesicle trafficking and lipid metabolism in synucleinopathy.Acta Neuropathol. 2021 Apr;141(4):491-510. doi: 10.1007/s00401-020-02177-z. Epub 2020 Jun 30. Acta Neuropathol. 2021. PMID: 32607605 Free PMC article. Review.
Cited by
-
Lysosomal Functions in Glia Associated with Neurodegeneration.Biomolecules. 2021 Mar 9;11(3):400. doi: 10.3390/biom11030400. Biomolecules. 2021. PMID: 33803137 Free PMC article. Review.
-
USP19 deubiquitinase inactivation regulates α-synuclein ubiquitination and inhibits accumulation of Lewy body-like aggregates in mice.NPJ Parkinsons Dis. 2023 Nov 28;9(1):157. doi: 10.1038/s41531-023-00601-1. NPJ Parkinsons Dis. 2023. PMID: 38017009 Free PMC article.
-
Signatures of glial activity can be detected in the CSF proteome.Proc Natl Acad Sci U S A. 2022 Jun 14;119(24):e2119804119. doi: 10.1073/pnas.2119804119. Epub 2022 Jun 6. Proc Natl Acad Sci U S A. 2022. PMID: 35666874 Free PMC article.
-
A multiverse of α-synuclein: investigation of prion strain properties with carboxyl-terminal truncation specific antibodies in animal models.Acta Neuropathol Commun. 2024 Jun 10;12(1):91. doi: 10.1186/s40478-024-01805-z. Acta Neuropathol Commun. 2024. PMID: 38858742 Free PMC article.
-
14-3-3 mitigates alpha-synuclein aggregation and toxicity in the in vivo preformed fibril model.Acta Neuropathol Commun. 2021 Jan 7;9(1):13. doi: 10.1186/s40478-020-01110-5. Acta Neuropathol Commun. 2021. PMID: 33413679 Free PMC article.
References
-
- Peelaerts W, Bousset L, Van Der Perren A, et al (2015) α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–344. 10.1038/nature14547 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

