Closing the brain-heart loop: Towards more holistic models of addiction and addiction recovery
- PMID: 32783345
- PMCID: PMC7878572
- DOI: 10.1111/adb.12958
Closing the brain-heart loop: Towards more holistic models of addiction and addiction recovery
Abstract
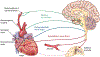
Much research seeks to articulate the brain structures and pathways implicated in addiction and addiction recovery. Prominent neurobiological models emphasize the interplay between cortical and limbic brain regions as a main driver of addictive processes, but largely do not take into consideration sensory and visceral information streams that link context and state to the brain and behavior. Yet these brain-body information streams would seem to be necessary elements of a comprehensive model of addiction. As a starting point, we describe the overlap between one current model of addiction circuitry and the neural network that not only regulates cardiovascular system activity but also receives feedback from peripheral cardiovascular processes through the baroreflex loop. We highlight the need for neurobiological, molecular, and behavioral studies of neural and peripheral cardiovascular signal integration during the experience of internal states and environmental contexts that drive alcohol and other drug use behaviors. We end with a call for systematic, mechanistic research on the promising, yet largely unexamined benefits to addiction treatment of neuroscience-informed, adjunctive interventions that target the malleability of the cardiovascular system to alter brain processes.
Keywords: autonomic nervous system; central autonomic network; substance use disorder.
©2020 Society for the Study of Addiction.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
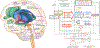
Similar articles
-
Sensory and motor aspects of addiction.Behav Brain Res. 2010 Mar 5;207(2):215-22. doi: 10.1016/j.bbr.2009.09.015. Epub 2009 Sep 12. Behav Brain Res. 2010. PMID: 19751771 Review.
-
Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex.Am J Psychiatry. 2002 Oct;159(10):1642-52. doi: 10.1176/appi.ajp.159.10.1642. Am J Psychiatry. 2002. PMID: 12359667 Free PMC article. Review.
-
Neurobiological Underpinnings in Drug Addiction.West Afr J Med. 2022 Aug 31;39(8):874-884. West Afr J Med. 2022. PMID: 36063103
-
The Genetically Informed Neurobiology of Addiction (GINA) model.Nat Rev Neurosci. 2023 Jan;24(1):40-57. doi: 10.1038/s41583-022-00656-8. Epub 2022 Nov 29. Nat Rev Neurosci. 2023. PMID: 36446900 Free PMC article. Review.
-
The role of interoception and alliesthesia in addiction.Pharmacol Biochem Behav. 2009 Nov;94(1):1-7. doi: 10.1016/j.pbb.2009.08.005. Epub 2009 Aug 19. Pharmacol Biochem Behav. 2009. PMID: 19698739 Free PMC article. Review.
Cited by
-
Time Since Last Drink is Positively Associated with Heart Rate Variability in Outpatients with Alcohol Use Disorder: Further Evidence of Psychophysiological Recovery in Early Alcohol Use Disorder Recovery.Appl Psychophysiol Biofeedback. 2023 Dec;48(4):433-437. doi: 10.1007/s10484-023-09597-z. Epub 2023 Jul 12. Appl Psychophysiol Biofeedback. 2023. PMID: 37436518
-
The crosstalk between fibroblast growth factor 21 (FGF21) system and substance use.iScience. 2024 Jun 25;27(7):110389. doi: 10.1016/j.isci.2024.110389. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055947 Free PMC article. Review.
-
Use and perceived usefulness of a just-in-time resonance breathing intervention adjunct for substance use disorder: Contextual and physiological predictors.Front Psychiatry. 2022 Sep 7;13:945751. doi: 10.3389/fpsyt.2022.945751. eCollection 2022. Front Psychiatry. 2022. PMID: 36159943 Free PMC article.
-
Reasons to be cheerful: Personal, civic, and economic achievements after resolving an alcohol or drug problem in the United States population.Psychol Addict Behav. 2021 Jun;35(4):402-414. doi: 10.1037/adb0000689. Epub 2021 Mar 25. Psychol Addict Behav. 2021. PMID: 33764087 Free PMC article.
-
Substance use and common contributors to morbidity: A genetics perspective.EBioMedicine. 2022 Sep;83:104212. doi: 10.1016/j.ebiom.2022.104212. Epub 2022 Aug 12. EBioMedicine. 2022. PMID: 35970022 Free PMC article. Review.
References
-
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4(1):1–32. - PubMed
-
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–1422. - PubMed
-
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. - PubMed
-
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996; 351(1346):1413–1420. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

