Overexpression of pyruvate dehydrogenase phosphatase 1 promotes the progression of pancreatic adenocarcinoma by regulating energy-related AMPK/mTOR signaling
- PMID: 32782783
- PMCID: PMC7412669
- DOI: 10.1186/s13578-020-00457-5
Overexpression of pyruvate dehydrogenase phosphatase 1 promotes the progression of pancreatic adenocarcinoma by regulating energy-related AMPK/mTOR signaling
Abstract
Background: Human pyruvate dehydrogenase phosphatase 1 (PDP1) plays an important physiological role in energy metabolism; however, its expression and function in human pancreatic adenocarcinoma (PDAC) remain unknown. This study aimed to investigate the expression pattern and mechanisms of action of PDP1 in human PDAC.
Methods: The expression pattern of PDP1 in PDAC was determined, and its correlation with patient survival was analyzed. Ectopic expression or knockdown of PDP1 was performed, and in vitro proliferation and migration, as well as in vivo tumor growth of PDAC, were measured. The mechanism was studied by biochemical approaches.
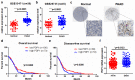
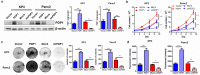
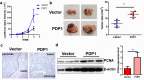
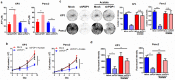
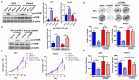
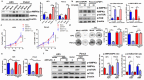
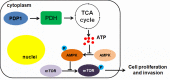
Results: PDP1 was overexpressed in human PDAC samples, and high expression of PDP1 correlated with poor overall and disease-free survival of PDAC patients. PDP1 promoted the proliferation, colony formation, and invasion of PDAC cells in vitro and facilitated orthotopic tumor growth in vivo. PDP1 accelerated intracellular ATP production, leading to sufficient energy to support rapid cancer progression. mTOR activation was responsible for the PDP1-induced tumor cell proliferation and invasion in PDAC. AMPK was downregulated by PDP1 overexpression, resulting in mTOR activation and cancer progression.
Conclusion: Our findings suggested that PDP1 could be a promising diagnostic and therapeutic target for anti-PDAC treatment.
Keywords: AMPK; ATP; Pancreatic adenocarcinoma; Pyruvate dehydrogenase phosphatase 1; mTOR.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
Similar articles
-
PDP1 promotes the progression of breast cancer through STAT3 pathway.Cell Biochem Funct. 2024 Apr;42(3):e3994. doi: 10.1002/cbf.3994. Cell Biochem Funct. 2024. PMID: 38566355
-
Tyr-94 phosphorylation inhibits pyruvate dehydrogenase phosphatase 1 and promotes tumor growth.J Biol Chem. 2014 Aug 1;289(31):21413-22. doi: 10.1074/jbc.M114.581124. Epub 2014 Jun 24. J Biol Chem. 2014. PMID: 24962578 Free PMC article.
-
Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4.J Exp Clin Cancer Res. 2018 Jul 3;37(1):130. doi: 10.1186/s13046-018-0807-2. J Exp Clin Cancer Res. 2018. PMID: 29970191 Free PMC article.
-
The lncRNA LINC01605 promotes the progression of pancreatic ductal adenocarcinoma by activating the mTOR signaling pathway.Cancer Cell Int. 2024 Jul 24;24(1):262. doi: 10.1186/s12935-024-03440-z. Cancer Cell Int. 2024. PMID: 39048994 Free PMC article.
-
Human Positive Coactivator 4 Affects the Progression and Prognosis of Pancreatic Ductal Adenocarcinoma via the mTOR/P70s6k Signaling Pathway.Onco Targets Ther. 2020 Nov 26;13:12213-12223. doi: 10.2147/OTT.S284219. eCollection 2020. Onco Targets Ther. 2020. PMID: 33273827 Free PMC article.
Cited by
-
Identification of shared gene signatures and pathways for diagnosing osteoporosis with sarcopenia through integrated bioinformatics analysis and machine learning.BMC Musculoskelet Disord. 2024 Jun 3;25(1):435. doi: 10.1186/s12891-024-07555-2. BMC Musculoskelet Disord. 2024. PMID: 38831425 Free PMC article.
-
MiR-18a-3p improves cartilage matrix remodeling and inhibits inflammation in osteoarthritis by suppressing PDP1.J Physiol Sci. 2022 Feb 11;72(1):3. doi: 10.1186/s12576-022-00827-3. J Physiol Sci. 2022. PMID: 35148687 Free PMC article.
-
NUSAP1 promotes pancreatic ductal adenocarcinoma progression by drives the epithelial-mesenchymal transition and reduces AMPK phosphorylation.BMC Cancer. 2024 Jan 16;24(1):87. doi: 10.1186/s12885-024-11842-5. BMC Cancer. 2024. PMID: 38229038 Free PMC article.
-
PDP1 Promotes Cell Malignant Behavior and Is Associated with Worse Clinical Features in Ovarian Cancer Patients: Evidence from Bioinformatics and In Vitro Level.Comput Math Methods Med. 2022 Oct 14;2022:7397250. doi: 10.1155/2022/7397250. eCollection 2022. Comput Math Methods Med. 2022. PMID: 36276992 Free PMC article.
-
PDP1 is a key metabolic gatekeeper and modulator of drug resistance in FLT3-ITD-positive acute myeloid leukemia.Leukemia. 2023 Dec;37(12):2367-2382. doi: 10.1038/s41375-023-02041-5. Epub 2023 Nov 7. Leukemia. 2023. PMID: 37935978 Free PMC article.
References
-
- Collaborators GBDPC The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):934–947. doi: 10.1016/S2468-1253(19)30347-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

