Not So Slim Anymore-Evidence for the Role of SUMO in the Regulation of Lipid Metabolism
- PMID: 32781719
- PMCID: PMC7466032
- DOI: 10.3390/biom10081154
Not So Slim Anymore-Evidence for the Role of SUMO in the Regulation of Lipid Metabolism
Abstract
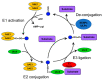
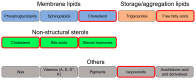
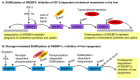
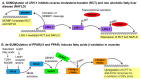
One of the basic building blocks of all life forms are lipids-biomolecules that dissolve in nonpolar organic solvents but not in water. Lipids have numerous structural, metabolic, and regulative functions in health and disease; thus, complex networks of enzymes coordinate the different compositions and functions of lipids with the physiology of the organism. One type of control on the activity of those enzymes is the conjugation of the Small Ubiquitin-like Modifier (SUMO) that in recent years has been identified as a critical regulator of many biological processes. In this review, I summarize the current knowledge about the role of SUMO in the regulation of lipid metabolism. In particular, I discuss (i) the role of SUMO in lipid metabolism of fungi and invertebrates; (ii) the function of SUMO as a regulator of lipid metabolism in mammals with emphasis on the two most well-characterized cases of SUMO regulation of lipid homeostasis. These include the effect of SUMO on the activity of two groups of master regulators of lipid metabolism-the Sterol Regulatory Element Binding Protein (SERBP) proteins and the family of nuclear receptors-and (iii) the role of SUMO as a regulator of lipid metabolism in arteriosclerosis, nonalcoholic fatty liver, cholestasis, and other lipid-related human diseases.
Keywords: SREBPs; SUMO; SUMO proteases; fatty acid metabolism; lipid metabolism; metabolism of cholesterol; nuclear receptors; steroid hormones.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Function and regulation of SUMO proteases.Nat Rev Mol Cell Biol. 2012 Dec;13(12):755-66. doi: 10.1038/nrm3478. Nat Rev Mol Cell Biol. 2012. PMID: 23175280 Free PMC article. Review.
-
The Roles of SUMO in Metabolic Regulation.Adv Exp Med Biol. 2017;963:143-168. doi: 10.1007/978-3-319-50044-7_9. Adv Exp Med Biol. 2017. PMID: 28197911 Free PMC article. Review.
-
SUMO Is a Critical Regulator of Salt Stress Responses in Rice.Plant Physiol. 2016 Apr;170(4):2378-91. doi: 10.1104/pp.15.01530. Epub 2016 Feb 11. Plant Physiol. 2016. PMID: 26869703 Free PMC article.
-
SUMOylation in the control of cholesterol homeostasis.Open Biol. 2020 May;10(5):200054. doi: 10.1098/rsob.200054. Epub 2020 May 6. Open Biol. 2020. PMID: 32370667 Free PMC article. Review.
-
E2-mediated small ubiquitin-like modifier (SUMO) modification of thymine DNA glycosylase is efficient but not selective for the enzyme-product complex.J Biol Chem. 2014 May 30;289(22):15810-9. doi: 10.1074/jbc.M114.572081. Epub 2014 Apr 21. J Biol Chem. 2014. PMID: 24753249 Free PMC article.
Cited by
-
Natural Products Against Renal Fibrosis via Modulation of SUMOylation.Front Pharmacol. 2022 Mar 4;13:800810. doi: 10.3389/fphar.2022.800810. eCollection 2022. Front Pharmacol. 2022. PMID: 35308200 Free PMC article. Review.
-
Roles of protein post-translational modifications in glucose and lipid metabolism: mechanisms and perspectives.Mol Med. 2023 Jul 6;29(1):93. doi: 10.1186/s10020-023-00684-9. Mol Med. 2023. PMID: 37415097 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

