Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration
- PMID: 32780530
- PMCID: PMC7820981
- DOI: 10.1111/bcpt.13478
Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration
Abstract
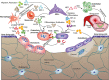
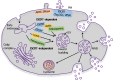
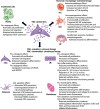
Mesenchymal stem cells (MSCs) and MSC-derived small extracellular vesicles (sEVs) are promising candidates for cell-based and cell-free regenerative medicine, respectively. By virtue of their multiple lineage differentiation capacity, MSCs have been implicated as an ideal tool for bone and cartilage regeneration. However, later observations attributed such regenerative effects to MSC-secreted paracrine factors. Exosomes, endosomal originated sEVs carrying lipid, protein and nucleic acid cargoes, were identified as components of the MSC secretome and propagated the key regenerative and immunoregulatory characteristics of parental MSCs. Here, exosome biogenesis, the molecular composition of exosomes, sEV-cell interactions and the effects on key bone homeostasis cells are reviewed. MSC-derived sEVs show to promote neovascularization and bone and cartilage regeneration in preclinical disease models. The mechanisms include the transfer of molecules, including microRNAs, mRNAs and proteins, to other key cells. MSC-derived sEVs are interesting candidates as biopharmaceuticals for drug delivery and for the engineering of biologically functionalized materials. Although major exploratory efforts have been made for therapeutic development, the secretion, distribution and biological effects of MSC-derived sEVs in bone and cartilage regeneration are not fully understood. Moreover, techniques for high-yield production, purity and storage need to be optimized before effective and safe MSC-derived sEVs therapies are realized.
Keywords: Bone regeneration; Cell-cell communication; Exosomes; Mesenchymal stem cells; small extracellular vesicles.
© 2020 The Authors. Basic & Clinical Pharmacology & Toxicology published by John Wiley & Sons Ltd on behalf of NordicAssociation for the Publication of BCPT (former Nordic Pharmacological Society).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Comparison of Curative Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells and Their Small Extracellular Vesicles in Treating Osteoarthritis.Int J Nanomedicine. 2021 Dec 16;16:8185-8202. doi: 10.2147/IJN.S336062. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34938076 Free PMC article.
-
Functionally engineered extracellular vesicles improve bone regeneration.Acta Biomater. 2020 Jun;109:182-194. doi: 10.1016/j.actbio.2020.04.017. Epub 2020 Apr 16. Acta Biomater. 2020. PMID: 32305445 Free PMC article.
-
Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway.Acta Biomater. 2022 Sep 15;150:413-426. doi: 10.1016/j.actbio.2022.07.015. Epub 2022 Jul 16. Acta Biomater. 2022. PMID: 35850484
-
MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment.Semin Cell Dev Biol. 2017 Jul;67:56-64. doi: 10.1016/j.semcdb.2016.11.008. Epub 2016 Nov 18. Semin Cell Dev Biol. 2017. PMID: 27871993 Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration.Cell Transplant. 2020 Jan-Dec;29:963689720908500. doi: 10.1177/0963689720908500. Cell Transplant. 2020. PMID: 32207341 Free PMC article. Review.
Cited by
-
Recent advances of CREKA peptide-based nanoplatforms in biomedical applications.J Nanobiotechnology. 2023 Mar 3;21(1):77. doi: 10.1186/s12951-023-01827-0. J Nanobiotechnology. 2023. PMID: 36869341 Free PMC article. Review.
-
The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings.Front Cell Dev Biol. 2021 Aug 18;9:661532. doi: 10.3389/fcell.2021.661532. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34490235 Free PMC article. Review.
-
Intrathecal Injection of Autologous Mesenchymal Stem-Cell-Derived Extracellular Vesicles in Spinal Cord Injury: A Feasibility Study in Pigs.Int J Mol Sci. 2023 May 4;24(9):8240. doi: 10.3390/ijms24098240. Int J Mol Sci. 2023. PMID: 37175946 Free PMC article.
-
Bone Mesenchymal Stem Cell-Derived sEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal miR-21.Front Bioeng Biotechnol. 2022 Jan 19;9:829136. doi: 10.3389/fbioe.2021.829136. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35127680 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review.Biomater Transl. 2022 Sep 28;3(3):175-187. doi: 10.12336/biomatertransl.2022.03.002. eCollection 2022. Biomater Transl. 2022. PMID: 36654775 Free PMC article. Review.
References
-
- DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthr Cartil. 2000;8(5):309‐334. - PubMed
-
- Elgali I. Molecular and structural patterns of guided bone regeneration (GBR). Gothenburg, Sweden: University of Gothenburg; 2015.
-
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641‐650. - PubMed
Publication types
MeSH terms
Grants and funding
- the Adlerbertska Foundation
- ALFGBG-725641/the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement
- 2018-02891/the Swedish Research Council
- the IngaBritt and Arne Lundberg Foundation
- the Area of Advance Materials of Chalmers/GU Biomaterials within the Strategic Research Area initiative launched by the Swedish government
LinkOut - more resources
Full Text Sources

