Impact of HIV-1 Vpr manipulation of the DNA repair enzyme UNG2 on B lymphocyte class switch recombination
- PMID: 32778120
- PMCID: PMC7418440
- DOI: 10.1186/s12967-020-02478-7
Impact of HIV-1 Vpr manipulation of the DNA repair enzyme UNG2 on B lymphocyte class switch recombination
Abstract
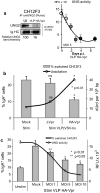
Background: HIV-1 Vpr encodes a 14 kDa protein that has been implicated in viral pathogenesis through modulation of several host cell functions. In addition to pro-apoptotic and cytostatic properties, Vpr can redirect cellular E3 ubiquitin ligases (such as DCAF1-Cul4A E3 ligase complex) to target many host proteins and interfere with their functions. Among them, Vpr binds the uracil DNA glycosylase UNG2, which controls genome uracilation, and induces its specific degradation leading to loss of uracil removal activity in infected cells. Considering the essential role of UNG2 in antibody diversification in B-cells, we evaluated the impact of Vpr on UNG2 fate in B lymphocytes and examined the functional consequences of UNG2 modulations on class switch recombination (CSR).
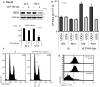
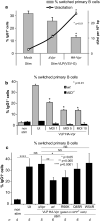
Methods: The impact of Vpr-induced UNG2 deregulation on CSR proficiency was evaluated by using virus-like particles able to deliver Vpr protein to target cells including the murine model CSR B cell line CH12F3 and mouse primary B-cells. Co-culture experiments were used to re-examine the ability of Vpr to be released by HIV-1 infected cells and to effectively accumulate in bystander B-cells. Vpr-mediated UNG2 modulations were monitored by following UNG2 protein abundance and uracil removal enzymatic activity.
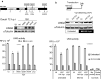
Results: In this study we report the ability of Vpr to reduce immunoglobulin class switch recombination (CSR) in immortalized and primary mouse B-cells through the degradation of UNG2. We also emphasize that Vpr is released by producing cells and penetrates bystander B lymphocytes.
Conclusions: This work therefore opens up new perspectives to study alterations of the B-cell response by using Vpr as a specific CSR blocking tool. Moreover, our results raise the question of whether extracellular HIV-1 Vpr detected in some patients may manipulate the antibody diversification process that engineers an adapted response against pathogenic intruders and thereby contribute to the intrinsic B-cell humoral defect reported in infected patients.
Keywords: Class switch recombination; Human immunodeficiency virus; Uracil DNA glycosylase 2; Uracilation; Vpr.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
HIV-1 Vpr loads uracil DNA glycosylase-2 onto DCAF1, a substrate recognition subunit of a cullin 4A-ring E3 ubiquitin ligase for proteasome-dependent degradation.J Biol Chem. 2010 Nov 26;285(48):37333-41. doi: 10.1074/jbc.M110.133181. Epub 2010 Sep 24. J Biol Chem. 2010. PMID: 20870715 Free PMC article.
-
The HIV1 protein Vpr acts to enhance constitutive DCAF1-dependent UNG2 turnover.PLoS One. 2012;7(1):e30939. doi: 10.1371/journal.pone.0030939. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22292079 Free PMC article.
-
Vpr expression abolishes the capacity of HIV-1 infected cells to repair uracilated DNA.Nucleic Acids Res. 2014 Feb;42(3):1698-710. doi: 10.1093/nar/gkt974. Epub 2013 Oct 30. Nucleic Acids Res. 2014. PMID: 24178031 Free PMC article.
-
Is Uracil-DNA Glycosylase UNG2 a New Cellular Weapon Against HIV-1?Curr HIV Res. 2019;17(3):148-160. doi: 10.2174/1570162X17666190821154331. Curr HIV Res. 2019. PMID: 31433761 Review.
-
DNA-uracil and human pathology.Mol Aspects Med. 2007 Jun-Aug;28(3-4):276-306. doi: 10.1016/j.mam.2007.04.006. Epub 2007 May 18. Mol Aspects Med. 2007. PMID: 17590428 Review.
Cited by
-
Caspase cleavage of gasdermin E causes neuronal pyroptosis in HIV-associated neurocognitive disorder.Brain. 2024 Feb 1;147(2):717-734. doi: 10.1093/brain/awad375. Brain. 2024. PMID: 37931057 Free PMC article.
-
Gastrointestinal germinal center B cell depletion and reduction in IgA+ plasma cells in HIV-1 infection.Sci Immunol. 2024 Oct 25;9(100):eado0090. doi: 10.1126/sciimmunol.ado0090. Epub 2024 Oct 25. Sci Immunol. 2024. PMID: 39454027 Free PMC article.
References
-
- Goh WC, et al. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. - PubMed
-
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. - PubMed
-
- Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998;273:13347–13352. - PubMed
-
- Agostini I, et al. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

