The Art and Science of Selecting a CD123-Specific Chimeric Antigen Receptor for Clinical Testing
- PMID: 32775492
- PMCID: PMC7393323
- DOI: 10.1016/j.omtm.2020.06.024
The Art and Science of Selecting a CD123-Specific Chimeric Antigen Receptor for Clinical Testing
Abstract
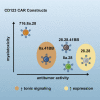
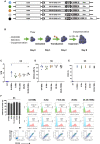
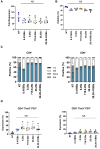
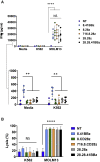
Chimeric antigen receptor (CAR) T cells targeting CD123, an acute myeloid leukemia (AML) antigen, hold the promise of improving outcomes for patients with refractory/recurrent disease. We generated five lentiviral vectors encoding CD20, which may serve as a target for CAR T cell depletion, and 2nd or 3rd generation CD123-CARs since the benefit of two costimulatory domains is model dependent. Four CARs were based on the CD123-specific single-chain variable fragment (scFv) 26292 (292) and one CAR on the CD123-specific scFv 26716 (716), respectively. We designed CARs with different hinge/transmembrane (H/TM) domains and costimulatory domains, in combination with the zeta (z) signaling domain: 292.CD8aH/TM.41BBz (8.41BBz), 292.CD8aH/TM.CD28z (8.28z), 716.CD8aH/TM.CD28z (716.8.28z), 292.CD28H/TM. CD28z (28.28z), and 292.CD28H/TM.CD28.41BBz (28.28.41BBz). Transduction efficiency, expansion, phenotype, and target cell recognition of the generated CD123-CAR T cells did not significantly differ. CAR constructs were eliminated for the following reasons: (1) 8.41BBz CARs induced significant baseline signaling, (2) 716.8.28z CAR T cells had decreased anti-AML activity, and (3) CD28.41BBz CAR T cells had no improved effector function in comparison to CD28z CAR T cells. We selected the 28.28z CAR since CAR expression on the cell surface of transduced T cells was higher in comparison to 8.28z CARs. The clinical study (NCT04318678) evaluating 28.28z CAR T cells is now open for patient accrual.
Keywords: AML; CAR; CD123; T cell therapy; chimeric antigen receptor; immunotherapy; leukemia.
© 2020 The Author(s).
Figures


Similar articles
-
Design and Assessment of Novel Anti-CD30 Chimeric Antigen Receptors with Human Antigen-Recognition Domains.Hum Gene Ther. 2021 Jul;32(13-14):730-743. doi: 10.1089/hum.2020.215. Epub 2021 Feb 22. Hum Gene Ther. 2021. PMID: 33287637 Free PMC article.
-
Comparative Evaluation of STEAP1 Targeting Chimeric Antigen Receptors with Different Costimulatory Domains and Spacers.Int J Mol Sci. 2024 Jan 2;25(1):586. doi: 10.3390/ijms25010586. Int J Mol Sci. 2024. PMID: 38203757 Free PMC article.
-
[Construction of a new anti-CD123 chimeric antigen receptor T cells and effect of anti-acute myeloid leukemia].Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 14;41(3):192-197. doi: 10.3760/cma.j.issn.0253-2727.2020.03.002. Zhonghua Xue Ye Xue Za Zhi. 2020. PMID: 32311887 Free PMC article. Chinese.
-
Structure of and Signalling Through Chimeric Antigen Receptor.2022 Feb 7. In: Kröger N, Gribben J, Chabannon C, Yakoub-Agha I, Einsele H, editors. The EBMT/EHA CAR-T Cell Handbook [Internet]. Cham (CH): Springer; 2022. Chapter 1. 2022 Feb 7. In: Kröger N, Gribben J, Chabannon C, Yakoub-Agha I, Einsele H, editors. The EBMT/EHA CAR-T Cell Handbook [Internet]. Cham (CH): Springer; 2022. Chapter 1. PMID: 36122078 Free Books & Documents. Review.
-
Challenges and Advances in Chimeric Antigen Receptor Therapy for Acute Myeloid Leukemia.Cancers (Basel). 2022 Jan 19;14(3):497. doi: 10.3390/cancers14030497. Cancers (Basel). 2022. PMID: 35158765 Free PMC article. Review.
Cited by
-
Integrome signatures of lentiviral gene therapy for SCID-X1 patients.Sci Adv. 2023 Oct 6;9(40):eadg9959. doi: 10.1126/sciadv.adg9959. Epub 2023 Oct 6. Sci Adv. 2023. PMID: 37801507 Free PMC article.
-
Recent Advances in Immune-Based Therapies for Acute Myeloid Leukemia.Blood Cancer Discov. 2024 Jul 1;5(4):234-248. doi: 10.1158/2643-3230.BCD-23-0202. Blood Cancer Discov. 2024. PMID: 38904305 Free PMC article. Review.
-
Immunotherapy in Acute Myeloid Leukemia: Where We Stand.Front Oncol. 2021 May 10;11:656218. doi: 10.3389/fonc.2021.656218. eCollection 2021. Front Oncol. 2021. PMID: 34041025 Free PMC article. Review.
-
Blastic plasmacytoid dendritic cell neoplasm: a comprehensive review in pediatrics, adolescents, and young adults (AYA) and an update of novel therapies.Leukemia. 2023 Sep;37(9):1767-1778. doi: 10.1038/s41375-023-01968-z. Epub 2023 Jul 14. Leukemia. 2023. PMID: 37452102 Free PMC article. Review.
-
High-Risk Acute Myeloid Leukemia: A Pediatric Prospective.Biomedicines. 2022 Jun 14;10(6):1405. doi: 10.3390/biomedicines10061405. Biomedicines. 2022. PMID: 35740427 Free PMC article. Review.
References
-
- Maude S.L. Tisagenlecleucel in pediatric patients with acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 2018;16:664–666. - PubMed
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

