Focus on the morphogenesis, fate and the role in tumor progression of multivesicular bodies
- PMID: 32771015
- PMCID: PMC7414566
- DOI: 10.1186/s12964-020-00619-5
Focus on the morphogenesis, fate and the role in tumor progression of multivesicular bodies
Abstract
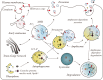
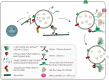
Multivesicular bodies (MVBs) are endosome organelles that are gradually attracting research attention. Initially, MVBs were considered as important components of the endosomal-lysosomal degradation pathway. In recent years, with an increase in extracellular vesicle (EV) research, the biogenesis, fate, and pathological effects of MVBs have been increasingly studied. However, the mechanisms by which MVBs are sorted to the lysosome and plasma membrane remain unclear. In addition, whether the trafficking of MVBs can determine whether exosomes are released from cells, the factors are involved in cargo loading and regulating the fate of MVBs, and the roles that MVBs play in the development of disease are unknown. Consequently, this review focuses on the mechanism of MVB biogenesis, intraluminal vesicle formation, sorting of different cargoes, and regulation of their fate. We also discuss the mechanisms of emerging amphisome-dependent secretion and degradation. In addition, we highlight the contributions of MVBs to the heterogeneity of EVs, and their important roles in cancer. Thus, we attempt to unravel the various functions of MVBs in the cell and their multiple roles in tumor progression. Video Abstract.
Keywords: Amphisome; Autophagy; Cancer; Extracellular vesicles; Multivesicular body; Release; Trafficking.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
The Obligate Intracellular Bacterial Pathogen Anaplasma phagocytophilum Exploits Host Cell Multivesicular Body Biogenesis for Proliferation and Dissemination.mBio. 2022 Dec 20;13(6):e0296122. doi: 10.1128/mbio.02961-22. Epub 2022 Nov 21. mBio. 2022. PMID: 36409075 Free PMC article.
-
Biogenesis of Plant Prevacuolar Multivesicular Bodies.Mol Plant. 2016 Jun 6;9(6):774-86. doi: 10.1016/j.molp.2016.01.011. Epub 2016 Feb 2. Mol Plant. 2016. PMID: 26836198 Review.
-
Multivesicular body morphogenesis.Annu Rev Cell Dev Biol. 2012;28:337-62. doi: 10.1146/annurev-cellbio-092910-154152. Epub 2012 Jul 20. Annu Rev Cell Dev Biol. 2012. PMID: 22831642 Review.
-
Multivesicular bodies in neurons: distribution, protein content, and trafficking functions.Prog Neurobiol. 2011 Mar;93(3):313-40. doi: 10.1016/j.pneurobio.2011.01.003. Epub 2011 Jan 7. Prog Neurobiol. 2011. PMID: 21216273 Free PMC article. Review.
-
A family of tetraspans organizes cargo for sorting into multivesicular bodies.Dev Cell. 2015 May 4;33(3):328-42. doi: 10.1016/j.devcel.2015.03.007. Dev Cell. 2015. PMID: 25942624 Free PMC article.
Cited by
-
The Biology of Lysosomes: From Order to Disorder.Biomedicines. 2023 Jan 14;11(1):213. doi: 10.3390/biomedicines11010213. Biomedicines. 2023. PMID: 36672721 Free PMC article. Review.
-
Circulating Exosome Cargoes Contain Functionally Diverse Cancer Biomarkers: From Biogenesis and Function to Purification and Potential Translational Utility.Cancers (Basel). 2022 Jul 10;14(14):3350. doi: 10.3390/cancers14143350. Cancers (Basel). 2022. PMID: 35884411 Free PMC article. Review.
-
Activation of the Wnt/β-catenin signalling pathway enhances exosome production by hucMSCs and improves their capability to promote diabetic wound healing.J Nanobiotechnology. 2024 Jun 26;22(1):373. doi: 10.1186/s12951-024-02650-x. J Nanobiotechnology. 2024. PMID: 38926800 Free PMC article.
-
Why Cells and Viruses Cannot Survive without an ESCRT.Cells. 2021 Feb 24;10(3):483. doi: 10.3390/cells10030483. Cells. 2021. PMID: 33668191 Free PMC article. Review.
-
Small but Mighty-Exosomes, Novel Intercellular Messengers in Neurodegeneration.Biology (Basel). 2022 Mar 8;11(3):413. doi: 10.3390/biology11030413. Biology (Basel). 2022. PMID: 35336787 Free PMC article. Review.
References
-
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

