Natural history of COVID-19 and current knowledge on treatment therapeutic options
- PMID: 32768971
- PMCID: PMC7332915
- DOI: 10.1016/j.biopha.2020.110493
Natural history of COVID-19 and current knowledge on treatment therapeutic options
Abstract
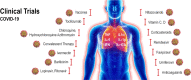
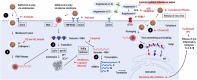
Despite intense research there is currently no effective vaccine available against the new severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged in the later 2019 and responsible for the COVID-19 pandemic. This infectious and communicable disease has become one of the major public health challenges in the world. The clinical management of COVID-19 has been limited to infection prevention and control measures associated with supportive care such as supplemental oxygen and mechanical ventilation. Meanwhile efforts to find an effective treatment to inhibit virus replication, mitigate the symptoms, increase survival and decrease mortality rate are ongoing. Several classes of drugs, many of them already in use for other diseases, are being evaluated based on the body of clinical knowledge obtained from infected patients regarding to the natural history and evolution of the infection. Herein we will provide an updated overview of the natural history and current knowledge on drugs and therapeutic agents being tested for the prevention and treatment of COVID-19. These include different classes of drugs such as antiviral agents (chloroquine, ivermectin, nitazoxanide, hydroxychloroquine, lopinavir, remdesivir, tocilizumab), supporting agents (Vitamin C, Vitamin D, azithromycin, corticosteroids) and promising investigational vaccines. Considering the controversies and excessive number of compounds being tested and reported in the literature we hope that this review can provide useful and updated consolidated information on potential drugs used to prevent, control and treat COVID-19 patients worldwide.
Keywords: Anakinra; Convalescent plasma; Corticosteroids; Hydroxychloroquine; SARS-CoV-2; Vaccine.
Copyright © 2020 The Author. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
There is no conflict of interest
Figures


Similar articles
-
Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2.Biomed Pharmacother. 2020 Nov;131:110668. doi: 10.1016/j.biopha.2020.110668. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861965 Free PMC article. Review.
-
Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised controlled trial (SEV-COVID Trial): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Oct 20;21(1):866. doi: 10.1186/s13063-020-04774-5. Trials. 2020. PMID: 33081849 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets.mBio. 2020 May 22;11(3):e01114-20. doi: 10.1128/mBio.01114-20. mBio. 2020. PMID: 32444382 Free PMC article.
-
Potential Antiviral Drugs for SARS-Cov-2 Treatment: Preclinical Findings and Ongoing Clinical Research.In Vivo. 2020 Jun;34(3 Suppl):1597-1602. doi: 10.21873/invivo.11949. In Vivo. 2020. PMID: 32503817 Free PMC article. Review.
Cited by
-
Associations of symptom combinations with in-hospital mortality of coronavirus disease-2019 patients using South Korean National data.PLoS One. 2022 Aug 26;17(8):e0273654. doi: 10.1371/journal.pone.0273654. eCollection 2022. PLoS One. 2022. PMID: 36018890 Free PMC article.
-
Fighting Omicron epidemic in China: Real-world big data from Fangcang shelter hospital during the outbreak in Shanghai 2022.J Infect. 2022 Oct;85(4):436-480. doi: 10.1016/j.jinf.2022.07.006. Epub 2022 Jul 12. J Infect. 2022. PMID: 35835412 Free PMC article. No abstract available.
-
Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases.Foods. 2023 Aug 30;12(17):3262. doi: 10.3390/foods12173262. Foods. 2023. PMID: 37685194 Free PMC article. Review.
-
Community responses to corona virus disease (COVID-19) in Africa in the face of "Infodemic": A scoping review.Parasite Epidemiol Control. 2024 Feb 27;25:e00345. doi: 10.1016/j.parepi.2024.e00345. eCollection 2024 May. Parasite Epidemiol Control. 2024. PMID: 38463547 Free PMC article. Review.
-
Evaluation of oxidative stress level: total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19.New Microbes New Infect. 2021 Jul;42:100897. doi: 10.1016/j.nmni.2021.100897. Epub 2021 May 17. New Microbes New Infect. 2021. PMID: 34026228 Free PMC article.
References
-
- World Health Organization. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...---12-march-2020. (accessed May 16, 2020).
-
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunubá Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020 Mar 30:S1473-3099(20)30243-7. doi: 10.1016/S1473-3099(20)30243-7. Epub ahead of print. Erratum in: Lancet Infect Dis. (2020) Apr 15;: Erratum in: Lancet Infect Dis. 2020 May 4;: PMID: 32240634; PMCID: PMC7158570. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

