Cellular poly(C) binding protein 2 interacts with porcine epidemic diarrhea virus papain-like protease 1 and supports viral replication
- PMID: 32768236
- PMCID: PMC7355335
- DOI: 10.1016/j.vetmic.2020.108793
Cellular poly(C) binding protein 2 interacts with porcine epidemic diarrhea virus papain-like protease 1 and supports viral replication
Abstract
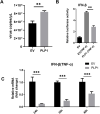
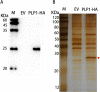
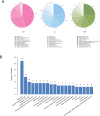
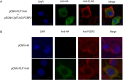
Porcine epidemic diarrhea virus (PEDV) belongs to the Alphacoronavirus genus in the Coronaviridae family. Similar to other coronaviruses, PEDV encodes two papain-like proteases. Papain-like protease (PLP)2 has been proposed to play a key role in antagonizing host innate immunity. However, the function of PLP1 remains unclear. In this study, we found that overexpression of PLP1 significantly promoted PEDV replication and inhibited production of interferon-β. Immunoprecipitation and mass spectrometry were used to identify cellular interaction partners of PLP1. Host cell poly(C) binding protein 2 (PCBP2) was determined to bind and interact with PLP1. Both endogenous and overexpressed PCBP2 co-localized with PLP1 in the cytoplasm. Overexpression of PLP1 upregulated expression of PCBP2. Furthermore, overexpression of PCBP2 promoted PEDV replication. Silencing of endogenous PCBP2 using small interfering RNAs attenuated PEDV replication. Taken together, these data demonstrated that PLP1 negatively regulated the production of type 1 interferon by interacting with PCBP2 and promoted PEDV replication.
Keywords: Coronavirus; Interaction; PEDV; Papain-like proteases; Poly(C) binding protein 2; Virus replication.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase.J Gen Virol. 2013 Jul;94(Pt 7):1554-1567. doi: 10.1099/vir.0.051169-0. Epub 2013 Apr 17. J Gen Virol. 2013. PMID: 23596270 Free PMC article.
-
Porcine Epidemic Diarrhea Virus Deficient in RNA Cap Guanine-N-7 Methylation Is Attenuated and Induces Higher Type I and III Interferon Responses.J Virol. 2020 Jul 30;94(16):e00447-20. doi: 10.1128/JVI.00447-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32461321 Free PMC article.
-
Coronavirus Porcine Epidemic Diarrhea Virus Nucleocapsid Protein Interacts with p53 To Induce Cell Cycle Arrest in S-Phase and Promotes Viral Replication.J Virol. 2021 Jul 26;95(16):e0018721. doi: 10.1128/JVI.00187-21. Epub 2021 Jul 26. J Virol. 2021. PMID: 34037422 Free PMC article.
-
Simian hemorrhagic fever virus: Recent advances.Virus Res. 2015 Apr 16;202:112-9. doi: 10.1016/j.virusres.2014.11.024. Epub 2014 Nov 29. Virus Res. 2015. PMID: 25455336 Free PMC article. Review.
-
Coronavirus accessory protein ORF3 biology and its contribution to viral behavior and pathogenesis.iScience. 2023 Apr 21;26(4):106280. doi: 10.1016/j.isci.2023.106280. Epub 2023 Feb 28. iScience. 2023. PMID: 36945252 Free PMC article. Review.
Cited by
-
The role of IBV PL1pro in virus replication and suppression of host innate immune responses.BMC Vet Res. 2023 Dec 13;19(1):270. doi: 10.1186/s12917-023-03839-2. BMC Vet Res. 2023. PMID: 38087313 Free PMC article.
-
Developing Next-Generation Live Attenuated Vaccines for Porcine Epidemic Diarrhea Using Reverse Genetic Techniques.Vaccines (Basel). 2024 May 19;12(5):557. doi: 10.3390/vaccines12050557. Vaccines (Basel). 2024. PMID: 38793808 Free PMC article. Review.
-
Regulation of cGAS/STING signaling and corresponding immune escape strategies of viruses.Front Cell Infect Microbiol. 2022 Sep 14;12:954581. doi: 10.3389/fcimb.2022.954581. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189363 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

