Respiration-Locking of Olfactory Receptor and Projection Neurons in the Mouse Olfactory Bulb and Its Modulation by Brain State
- PMID: 32765224
- PMCID: PMC7378796
- DOI: 10.3389/fncel.2020.00220
Respiration-Locking of Olfactory Receptor and Projection Neurons in the Mouse Olfactory Bulb and Its Modulation by Brain State
Abstract
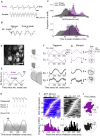
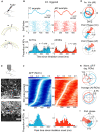
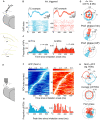
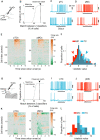
For sensory systems of the brain, the dynamics of an animal's own sampling behavior has a direct consequence on ensuing computations. This is particularly the case for mammalian olfaction, where a rhythmic flow of air over the nasal epithelium entrains activity in olfactory system neurons in a phenomenon known as sniff-locking. Parameters of sniffing can, however, change drastically with brain states. Coupled to the fact that different observation methods have different kinetics, consensus on the sniff-locking properties of neurons is lacking. To address this, we investigated the sniff-related activity of olfactory sensory neurons (OSNs), as well as the principal neurons of the olfactory bulb (OB), using 2-photon calcium imaging and intracellular whole-cell patch-clamp recordings in vivo, both in anesthetized and awake mice. Our results indicate that OSNs and OB output neurons lock robustly to the sniff rhythm, but with a slight temporal shift between behavioral states. We also observed a slight delay between methods. Further, the divergent sniff-locking by tufted cells (TCs) and mitral cells (MCs) in the absence of odor can be used to determine the cell type reliably using a simple linear classifier. Using this classification on datasets where morphological identification is unavailable, we find that MCs use a wider range of temporal shifts to encode odors than previously thought, while TCs have a constrained timing of activation due to an early-onset hyperpolarization. We conclude that the sniff rhythm serves as a fundamental rhythm but its impact on odor encoding depends on cell type, and this difference is accentuated in awake mice.
Keywords: active sampling; electrophysiology; imaging; olfaction; olfactory bulb; temporal coding.
Copyright © 2020 Ackels, Jordan, Schaefer and Fukunaga.
Figures

Similar articles
-
Differential Impacts of Repeated Sampling on Odor Representations by Genetically-Defined Mitral and Tufted Cell Subpopulations in the Mouse Olfactory Bulb.J Neurosci. 2020 Aug 5;40(32):6177-6188. doi: 10.1523/JNEUROSCI.0258-20.2020. Epub 2020 Jun 29. J Neurosci. 2020. PMID: 32601245 Free PMC article.
-
Inhalation Frequency Controls Reformatting of Mitral/Tufted Cell Odor Representations in the Olfactory Bulb.J Neurosci. 2018 Feb 28;38(9):2189-2206. doi: 10.1523/JNEUROSCI.0714-17.2018. Epub 2018 Jan 26. J Neurosci. 2018. PMID: 29374137 Free PMC article.
-
Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb.J Neurosci. 2011 Jul 20;31(29):10615-26. doi: 10.1523/JNEUROSCI.1805-11.2011. J Neurosci. 2011. PMID: 21775605 Free PMC article.
-
The sniff as a unit of olfactory processing.Chem Senses. 2006 Feb;31(2):167-79. doi: 10.1093/chemse/bjj016. Epub 2005 Dec 8. Chem Senses. 2006. PMID: 16339265 Review.
-
Performance of a Computational Model of the Mammalian Olfactory System.In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 6. In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 6. PMID: 26042330 Free Books & Documents. Review.
Cited by
-
How the sense of smell influences cognition throughout life.Neuroforum. 2022 Aug 26;28(3):177-185. doi: 10.1515/nf-2022-0007. Epub 2022 Jun 6. Neuroforum. 2022. PMID: 36067120 Free PMC article. Review.
-
How do mammals convert dynamic odor information into neural maps for landscape navigation?PLoS Biol. 2024 Nov 21;22(11):e3002908. doi: 10.1371/journal.pbio.3002908. eCollection 2024 Nov. PLoS Biol. 2024. PMID: 39571004 Free PMC article.
-
Nasal airflow engages central olfactory processing and shapes olfactory percepts.Proc Biol Sci. 2020 Oct 28;287(1937):20201772. doi: 10.1098/rspb.2020.1772. Epub 2020 Oct 28. Proc Biol Sci. 2020. PMID: 33109009 Free PMC article.
-
Generation and Characterization of a Cell Type-Specific, Inducible Cre-Driver Line to Study Olfactory Processing.J Neurosci. 2021 Jul 28;41(30):6449-6467. doi: 10.1523/JNEUROSCI.3076-20.2021. Epub 2021 Jun 7. J Neurosci. 2021. PMID: 34099512 Free PMC article.
-
Sample Preparation and Warping Accuracy for Correlative Multimodal Imaging in the Mouse Olfactory Bulb Using 2-Photon, Synchrotron X-Ray and Volume Electron Microscopy.Front Cell Dev Biol. 2022 Jun 8;10:880696. doi: 10.3389/fcell.2022.880696. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35756997 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

