LncRNA PLAC2 Positively Regulates CDK2 to Promote Ovarian Carcinoma Cell Proliferation
- PMID: 32765074
- PMCID: PMC7367733
- DOI: 10.2147/CMAR.S242781
LncRNA PLAC2 Positively Regulates CDK2 to Promote Ovarian Carcinoma Cell Proliferation
Abstract
Background: PLAC2 has been reported to participate in glioma, but its role in ovarian carcinoma (OC) is unclear. This study investigated the role of lncRNA PLAC2 in OC.
Methods: A 5-year follow-up study of 64 patients was carried out in Weihai Municipal Hospital after the admission of patients. A total of 64 OC patients were selected from 178 OC patients admitted in the aforementioned hospital from August 2011 to January 2014. Cell transfections, cell cycle analysis, cell proliferation assay and Western blot were carried out during the research.
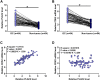
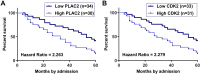
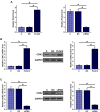
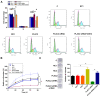
Results: The expression levels of PLAC2 and CDK2 were both upregulated in OC and they were positively correlated. During the 5-year follow-up, patients with high levels of PLAC2 and CDK2 showed significantly lower overall survival rate. In OC cells, overexpression of PLAC2 resulted in upregulated, while silencing of PLAC2 resulted in downregulated expression of CDK2. Cell proliferation assay showed that overexpression of PLAC2 resulted in increased, while silencing of PLAC2 resulted in decreased proliferation rate of OC cells. In addition, overexpression of CDK2 attenuated the effects of silencing of PLAC2.
Conclusion: PLAC2 positively regulates CDK2 to promote OC cell proliferation.
Keywords: CDK2; PLAC2; ovarian carcinoma; survival.
© 2020 He et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
LncRNA PLAC 2 downregulated miR-21 in non-small cell lung cancer and predicted survival.BMC Pulm Med. 2019 Sep 10;19(1):172. doi: 10.1186/s12890-019-0931-6. BMC Pulm Med. 2019. PMID: 31500623 Free PMC article.
-
LncRNA PLAC2 upregulates p53 to induce hepatocellular carcinoma cell apoptosis.Gene. 2019 Sep 5;712:143944. doi: 10.1016/j.gene.2019.143944. Epub 2019 Jun 21. Gene. 2019. PMID: 31233763
-
Long noncoding RNA PLAC2 regulates PTEN in retinoblastoma and participates in the regulation of cancer cell apoptosis.Oncol Lett. 2020 Mar;19(3):2489-2494. doi: 10.3892/ol.2020.11314. Epub 2020 Jan 17. Oncol Lett. 2020. PMID: 32194749 Free PMC article.
-
lncRNA PLAC2 activated by H3K27 acetylation promotes cell proliferation and invasion via the activation of Wnt/β‑catenin pathway in oral squamous cell carcinoma.Int J Oncol. 2019 Apr;54(4):1183-1194. doi: 10.3892/ijo.2019.4707. Epub 2019 Feb 1. Int J Oncol. 2019. PMID: 30720068 Free PMC article.
-
The Long Non-Coding RNA (lncRNA) AGAP2-AS1 is Upregulated in Ovarian Carcinoma and Negatively Regulates lncRNA MEG3.Med Sci Monit. 2019 Jun 24;25:4699-4704. doi: 10.12659/MSM.914766. Med Sci Monit. 2019. PMID: 31233485 Free PMC article.
Cited by
-
LncRNA CRNDE promotes cell proliferation, migration and invasion of ovarian cancer via miR-423-5p/FSCN1 axis.Mol Cell Biochem. 2022 May;477(5):1477-1488. doi: 10.1007/s11010-022-04382-8. Epub 2022 Feb 15. Mol Cell Biochem. 2022. PMID: 35166986
-
A review on the role of cyclin dependent kinases in cancers.Cancer Cell Int. 2022 Oct 20;22(1):325. doi: 10.1186/s12935-022-02747-z. Cancer Cell Int. 2022. PMID: 36266723 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

