Small Molecule Adjuvants Potentiate Colistin Activity and Attenuate Resistance Development in Escherichia coli by Affecting pmr AB System
- PMID: 32764996
- PMCID: PMC7360418
- DOI: 10.2147/IDR.S260766
Small Molecule Adjuvants Potentiate Colistin Activity and Attenuate Resistance Development in Escherichia coli by Affecting pmr AB System
Abstract
Background: Colistin is one of the last-resort antibiotics to treat multi-drug resistant (MDR) Gram-negative bacterial infections in humans. Further, colistin has been also used to prevent and treat Enterobacteriaceae infections in food animals. However, chromosomal mutations and mobile colistin resistance (mcr) genes, which confer resistance to colistin, have been detected in bacterial isolates from food animals and humans worldwide; thus, limiting the use of colistin. Therefore, strategies that could aid in ameliorating colistin resistance are critically needed.
Objective: Investigate the adjuvant potential of novel small molecules (SMs) on colistin.
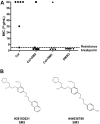
Materials and methods: Previously, we identified 11 membrane-affecting SMs with bactericidal activity against avian pathogenic Escherichia coli (APEC). Here, we investigated the potentiation effect of those SMs on colistin using checkerboard assays and wax moth (Galleria mellonella) larval model. The impact of the SM combination on colistin resistance evolution was also investigated by analyzing whole genome sequences of APEC isolates passaged with colistin alone or in combination with SMs followed by quantitating pmrCAB and pmrH expression in those isolates.
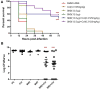
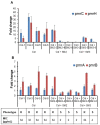
Results: The SM combination synergistically reduced the minimum bactericidal concentration of colistin by at least 10-fold. In larvae, the SM combination increased the efficacy of colistin by two-fold with enhanced (>50%) survival and reduced (>4 logs) APEC load. Further, the SM combination decreased the frequency (5/6 to 1/6) of colistin resistance evolution and downregulated the pmrCAB and pmrH expression. Previously unknown mutations in pmrB (L14Q, T92P) and pmrA (A80V), which were predicted deleterious, were identified in the colistin-resistant (ColR) APEC isolates when passaged with colistin alone but not in combination with SMs. Our study also identified mutations in hypothetical and several phage-related proteins in ColR APEC isolates in concurrent with pmrAB mutations.
Conclusion: Our study identified two SMs (SM2 and SM3) that potentiated the colistin activity and attenuated the development of colistin resistance in APEC. These SMs can be developed as anti-evolution drugs that can slow down colistin resistance development.
Keywords: anti-evolution drug; colistin; pmrCAB; pmrH; resistance; small molecules.
© 2020 Kathayat et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Contribution of Novel Amino Acid Alterations in PmrA or PmrB to Colistin Resistance in mcr-Negative Escherichia coli Clinical Isolates, Including Major Multidrug-Resistant Lineages O25b:H4-ST131-H30Rx and Non-x.Antimicrob Agents Chemother. 2018 Aug 27;62(9):e00864-18. doi: 10.1128/AAC.00864-18. Print 2018 Sep. Antimicrob Agents Chemother. 2018. PMID: 29914952 Free PMC article.
-
PmrB mutations including a novel 10-amino acid repeat sequence insertion associated with low-level colistin resistance in carbapenem-resistant Acinetobacter baumannii.Infect Genet Evol. 2020 Nov;85:104577. doi: 10.1016/j.meegid.2020.104577. Epub 2020 Sep 30. Infect Genet Evol. 2020. PMID: 33007498
-
Sequence Duplication Within pmrB Gene Contribute to High-Level Colistin Resistance in Avian Pathogenic Escherichia coli.Microb Drug Resist. 2020 Dec;26(12):1442-1451. doi: 10.1089/mdr.2019.0290. Epub 2019 Nov 26. Microb Drug Resist. 2020. PMID: 31770069
-
Acquisition of the mcr-1 Gene Lowers the Target Mutation to Impede the Evolution of a High-Level Colistin-Resistant Mutant in Escherichia coli.Infect Drug Resist. 2021 Aug 10;14:3041-3051. doi: 10.2147/IDR.S324303. eCollection 2021. Infect Drug Resist. 2021. PMID: 34408448 Free PMC article.
-
Current Update on Intrinsic and Acquired Colistin Resistance Mechanisms in Bacteria.Front Med (Lausanne). 2021 Aug 12;8:677720. doi: 10.3389/fmed.2021.677720. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34476235 Free PMC article. Review.
Cited by
-
The synergistic antibacterial activity and mechanism of colistin-oxethazaine combination against gram-negative pathogens.Front Pharmacol. 2024 Mar 21;15:1363441. doi: 10.3389/fphar.2024.1363441. eCollection 2024. Front Pharmacol. 2024. PMID: 38576480 Free PMC article.
-
Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens.Antibiotics (Basel). 2023 Jan 30;12(2):274. doi: 10.3390/antibiotics12020274. Antibiotics (Basel). 2023. PMID: 36830185 Free PMC article. Review.
-
Novel Small Molecule Growth Inhibitor Affecting Bacterial Outer Membrane Reduces Extraintestinal Pathogenic Escherichia coli (ExPEC) Infection in Avian Model.Microbiol Spectr. 2021 Oct 31;9(2):e0000621. doi: 10.1128/Spectrum.00006-21. Epub 2021 Sep 1. Microbiol Spectr. 2021. PMID: 34468186 Free PMC article.
-
Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies.Pathogens. 2021 Apr 12;10(4):467. doi: 10.3390/pathogens10040467. Pathogens. 2021. PMID: 33921518 Free PMC article. Review.
-
Global colistin use: a review of the emergence of resistant Enterobacterales and the impact on their genetic basis.FEMS Microbiol Rev. 2022 Feb 9;46(1):fuab049. doi: 10.1093/femsre/fuab049. FEMS Microbiol Rev. 2022. PMID: 34612488 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

