Modulation of glycosyltransferase ST6Gal-I in gastric cancer-derived organoids disrupts homeostatic epithelial cell turnover
- PMID: 32763973
- PMCID: PMC7549044
- DOI: 10.1074/jbc.RA120.014887
Modulation of glycosyltransferase ST6Gal-I in gastric cancer-derived organoids disrupts homeostatic epithelial cell turnover
Abstract
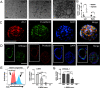
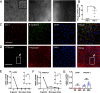
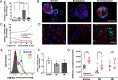
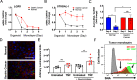
Programmed cell death promotes homeostatic cell turnover in the epithelium but is dysregulated in cancer. The glycosyltransferase ST6Gal-I is known to block homeostatic apoptosis through α2,6-linked sialylation of the death receptor TNFR1 in many cell types. However, its role has not been investigated in gastric epithelial cells or gastric tumorigenesis. We determined that human gastric antral epithelium rarely expressed ST6Gal-I, but the number of ST6Gal-I-expressing epithelial cells increased significantly with advancing premalignancy leading to cancer. The mRNA expression levels of ST6GAL-I and SOX9 in human gastric epithelial cells correlated positively with one another through the premalignancy cascade, indicating that increased epithelial cell expression of ST6Gal-I is associated with premalignant progression. To determine the functional impact of increased ST6Gal-I, we generated human gastric antral organoids from epithelial stem cells and differentiated epithelial monolayers from gastric organoids. Gastric epithelial stem cells strongly expressed ST6Gal-I, suggesting a novel biomarker of stemness. In contrast, organoid-derived epithelial monolayers expressed markedly reduced ST6Gal-I and underwent TNF-induced, caspase-mediated apoptosis, consistent with homeostasis. Conversely, epithelial monolayers generated from gastric cancer stem cells retained high levels of ST6Gal-I and resisted TNF-induced apoptosis, supporting prolonged survival. Protection from TNF-induced apoptosis depended on ST6Gal-I overexpression, because forced ST6Gal-I overexpression in normal gastric stem cell-differentiated monolayers inhibited TNF-induced apoptosis, and cleavage of α2,6-linked sialic acids from gastric cancer organoid-derived monolayers restored susceptibility to TNF-induced apoptosis. These findings implicate up-regulated ST6Gal-I expression in blocking homeostatic epithelial cell apoptosis in gastric cancer pathogenesis, suggesting a mechanism for prolonged epithelioid tumor cell survival.
Keywords: apoptosis; cancer biology; epithelial cell; gastric cancer; glycosylation; sialic acid; stem cells; stem cellsα-26-sialyltransferase 1 (ST6Gal-I); β-galactoside α2,6-sialyltransferase 1 (ST6GAL1).
© 2020 Alexander et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
The Tumor-Associated Glycosyltransferase ST6Gal-I Regulates Stem Cell Transcription Factors and Confers a Cancer Stem Cell Phenotype.Cancer Res. 2016 Jul 1;76(13):3978-88. doi: 10.1158/0008-5472.CAN-15-2834. Epub 2016 May 23. Cancer Res. 2016. PMID: 27216178 Free PMC article.
-
ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor.J Biol Chem. 2018 Feb 2;293(5):1610-1622. doi: 10.1074/jbc.M117.801480. Epub 2017 Dec 12. J Biol Chem. 2018. PMID: 29233887 Free PMC article.
-
The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1α signaling.J Biol Chem. 2018 Apr 13;293(15):5659-5667. doi: 10.1074/jbc.RA117.001194. Epub 2018 Feb 23. J Biol Chem. 2018. PMID: 29475939 Free PMC article.
-
Significance of β-Galactoside α2,6 Sialyltranferase 1 in Cancers.Molecules. 2015 Apr 24;20(5):7509-27. doi: 10.3390/molecules20057509. Molecules. 2015. PMID: 25919275 Free PMC article. Review.
-
Stem cells in tissues, organoids, and cancers.Cell Mol Life Sci. 2019 Oct;76(20):4043-4070. doi: 10.1007/s00018-019-03199-x. Epub 2019 Jul 17. Cell Mol Life Sci. 2019. PMID: 31317205 Free PMC article. Review.
Cited by
-
Tumor organoids: applications in cancer modeling and potentials in precision medicine.J Hematol Oncol. 2022 May 12;15(1):58. doi: 10.1186/s13045-022-01278-4. J Hematol Oncol. 2022. PMID: 35551634 Free PMC article. Review.
-
Aberrant Sialylation in Cancer: Biomarker and Potential Target for Therapeutic Intervention?Cancers (Basel). 2021 Apr 22;13(9):2014. doi: 10.3390/cancers13092014. Cancers (Basel). 2021. PMID: 33921986 Free PMC article. Review.
-
Paligenosis: Cellular Remodeling During Tissue Repair.Annu Rev Physiol. 2022 Feb 10;84:461-483. doi: 10.1146/annurev-physiol-061121-035954. Epub 2021 Oct 27. Annu Rev Physiol. 2022. PMID: 34705482 Free PMC article. Review.
-
ST6GAL1 sialyltransferase promotes acinar to ductal metaplasia and pancreatic cancer progression.JCI Insight. 2023 Oct 9;8(19):e161563. doi: 10.1172/jci.insight.161563. JCI Insight. 2023. PMID: 37643018 Free PMC article.
-
ST6Gal1: Oncogenic signaling pathways and targets.Front Mol Biosci. 2022 Aug 29;9:962908. doi: 10.3389/fmolb.2022.962908. eCollection 2022. Front Mol Biosci. 2022. PMID: 36106023 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

