Distinct Phases of Postnatal Skeletal Muscle Growth Govern the Progressive Establishment of Muscle Stem Cell Quiescence
- PMID: 32763161
- PMCID: PMC7486220
- DOI: 10.1016/j.stemcr.2020.07.011
Distinct Phases of Postnatal Skeletal Muscle Growth Govern the Progressive Establishment of Muscle Stem Cell Quiescence
Abstract
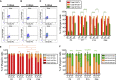
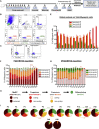
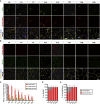
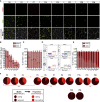
Muscle stem cells (or muscle satellite cells [MuSCs]) are required for postnatal growth. Yet, the detailed characterization of myogenic progression and establishment of quiescence during this process remains poorly documented. Here, we provide an overview of myogenic cells heterogeneity and dynamic from birth to adulthood using flow cytometry. We demonstrated that PAX7+ cells acquire an increasing ability to progress in the myogenic program from birth to adulthood. We then simultaneously analyzed the cycling state (KI67 expression) of the MuSCs and progenitors (PAX7+) and their progression into myogenic precursors (PAX7-MYOD+) and differentiating cells (MYOG+) in vivo. We identified two distinct peaks of myogenic differentiation between P7-P10 and P21-P28, and showed that the quiescent MuSC pool is established between 7 and 8 weeks of age. Overall our study provides a comprehensive in vivo characterization of myogenic heterogeneity and demonstrates the highly dynamic nature of skeletal muscle postnatal growth process.
Keywords: muscle satellite cells; muscle stem cells; myogenesis; myogenic precursors; myogenic progenitors; postnatal growth; quiescence.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development.Nat Cell Biol. 2010 Mar;12(3):257-66. doi: 10.1038/ncb2025. Epub 2010 Jan 31. Nat Cell Biol. 2010. PMID: 20118923
-
Isolation of progenitors that exhibit myogenic/osteogenic bipotency in vitro by fluorescence-activated cell sorting from human fetal muscle.Stem Cell Reports. 2014 Jan 14;2(1):92-106. doi: 10.1016/j.stemcr.2013.12.006. eCollection 2014 Jan 14. Stem Cell Reports. 2014. PMID: 24678452 Free PMC article.
-
Established cell surface markers efficiently isolate highly overlapping populations of skeletal muscle satellite cells by fluorescence-activated cell sorting.Skelet Muscle. 2016 Nov 8;6:35. doi: 10.1186/s13395-016-0106-6. eCollection 2016. Skelet Muscle. 2016. PMID: 27826411 Free PMC article.
-
From cyclins to CDKIs: Cell cycle regulation of skeletal muscle stem cell quiescence and activation.Exp Cell Res. 2022 Nov 1;420(1):113275. doi: 10.1016/j.yexcr.2022.113275. Epub 2022 Aug 2. Exp Cell Res. 2022. PMID: 35931143 Review.
-
Muscle stem cells as immunomodulator during regeneration.Curr Top Dev Biol. 2024;158:221-238. doi: 10.1016/bs.ctdb.2024.01.010. Epub 2024 Feb 15. Curr Top Dev Biol. 2024. PMID: 38670707 Review.
Cited by
-
Chemoradiation impairs myofiber hypertrophic growth in a pediatric tumor model.Sci Rep. 2020 Nov 11;10(1):19501. doi: 10.1038/s41598-020-75913-w. Sci Rep. 2020. PMID: 33177579 Free PMC article.
-
Aging of the immune system and impaired muscle regeneration: A failure of immunomodulation of adult myogenesis.Exp Gerontol. 2021 Mar;145:111200. doi: 10.1016/j.exger.2020.111200. Epub 2020 Dec 24. Exp Gerontol. 2021. PMID: 33359378 Free PMC article. Review.
-
The muscle stem cell niche at a glance.J Cell Sci. 2023 Dec 15;136(24):jcs261200. doi: 10.1242/jcs.261200. Epub 2023 Dec 27. J Cell Sci. 2023. PMID: 38149870 Free PMC article.
-
The asymmetrical ESR1 signaling in muscle progenitor cells determines the progression of adolescent idiopathic scoliosis.Cell Discov. 2023 Apr 25;9(1):44. doi: 10.1038/s41421-023-00531-5. Cell Discov. 2023. PMID: 37185898 Free PMC article.
-
Progressive and Coordinated Mobilization of the Skeletal Muscle Niche throughout Tissue Repair Revealed by Single-Cell Proteomic Analysis.Cells. 2021 Mar 28;10(4):744. doi: 10.3390/cells10040744. Cells. 2021. PMID: 33800595 Free PMC article.
References
-
- Biressi S., Molinaro M., Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev. Biol. 2007;308:281–293. - PubMed
-
- Bröhl D., Vasyutina E., Czajkowski M.T., Griger J., Rassek C., Rahn H.-P., Purfürst B., Wende H., Birchmeier C. Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on notch signals. Dev. Cell. 2012;23:469–481. - PubMed
-
- Chargé S.B.P., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

