Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease
- PMID: 32760286
- PMCID: PMC7373076
- DOI: 10.3389/fphys.2020.00755
Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease
Abstract
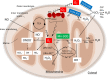
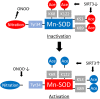
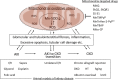
The mitochondria are a major source of reactive oxygen species (ROS). Superoxide anion (O2 •-) is produced by the process of oxidative phosphorylation associated with glucose, amino acid, and fatty acid metabolism, resulting in the production of adenosine triphosphate (ATP) in the mitochondria. Excess production of reactive oxidants in the mitochondria, including O2 •-, and its by-product, peroxynitrite (ONOO-), which is generated by a reaction between O2 •- with nitric oxide (NO•), alters cellular function via oxidative modification of proteins, lipids, and nucleic acids. Mitochondria maintain an antioxidant enzyme system that eliminates excess ROS; manganese superoxide dismutase (Mn-SOD) is one of the major components of this system, as it catalyzes the first step involved in scavenging ROS. Reduced expression and/or the activity of Mn-SOD results in diminished mitochondrial antioxidant capacity; this can impair the overall health of the cell by altering mitochondrial function and may lead to the development and progression of kidney disease. Targeted therapeutic agents may protect mitochondrial proteins, including Mn-SOD against oxidative stress-induced dysfunction, and this may consequently lead to the protection of renal function. Here, we describe the biological function and regulation of Mn-SOD and review the significance of mitochondrial oxidative stress concerning the pathogenesis of kidney diseases, including chronic kidney disease (CKD) and acute kidney injury (AKI), with a focus on Mn-SOD dysfunction.
Keywords: acute kidney injury; chronic kidney disease; manganese superoxide dismutase; mitochondria; peroxynitrite; posttranslational modification.
Copyright © 2020 Kitada, Xu, Ogura, Monno and Koya.
Figures
Similar articles
-
Mitochondrial superoxide mediates labile iron level: evidence from Mn-SOD-transgenic mice and heterozygous knockout mice and isolated rat liver mitochondria.Free Radic Biol Med. 2013 Dec;65:143-149. doi: 10.1016/j.freeradbiomed.2013.06.026. Epub 2013 Jun 20. Free Radic Biol Med. 2013. PMID: 23792772
-
Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease.Redox Biol. 2019 Jan;20:98-106. doi: 10.1016/j.redox.2018.09.020. Epub 2018 Sep 27. Redox Biol. 2019. PMID: 30296702 Free PMC article.
-
A mitochondrial superoxide theory for oxidative stress diseases and aging.J Clin Biochem Nutr. 2015 Jan;56(1):1-7. doi: 10.3164/jcbn.14-42. Epub 2014 Dec 23. J Clin Biochem Nutr. 2015. PMID: 25834301 Free PMC article. Review.
-
Distribution of mitochondrial manganese superoxide dismutase among rat glial cells in culture.Glia. 1998 Apr;22(4):408-14. Glia. 1998. PMID: 9517573
-
Manganese superoxide dismutase: guardian of the powerhouse.Int J Mol Sci. 2011;12(10):7114-62. doi: 10.3390/ijms12107114. Epub 2011 Oct 21. Int J Mol Sci. 2011. PMID: 22072939 Free PMC article. Review.
Cited by
-
Superoxide Dismutase Administration: A Review of Proposed Human Uses.Molecules. 2021 Mar 25;26(7):1844. doi: 10.3390/molecules26071844. Molecules. 2021. PMID: 33805942 Free PMC article.
-
The association of manganese levels with red cell distribution width: A population-based study.PLoS One. 2024 Aug 15;19(8):e0292569. doi: 10.1371/journal.pone.0292569. eCollection 2024. PLoS One. 2024. PMID: 39146304 Free PMC article.
-
Assessing Curcumin Uptake and Clearance and Their Influence on Superoxide Dismutase Activity in Drosophila melanogaster.BioTech (Basel). 2023 Sep 8;12(3):58. doi: 10.3390/biotech12030058. BioTech (Basel). 2023. PMID: 37754202 Free PMC article.
-
Catalytic Antioxidants in the Kidney.Antioxidants (Basel). 2021 Jan 18;10(1):130. doi: 10.3390/antiox10010130. Antioxidants (Basel). 2021. PMID: 33477607 Free PMC article. Review.
-
Manganese-Implanted Titanium Modulates the Crosstalk between Bone Marrow Mesenchymal Stem Cells and Macrophages to Improve Osteogenesis.J Funct Biomater. 2023 Sep 3;14(9):456. doi: 10.3390/jfb14090456. J Funct Biomater. 2023. PMID: 37754870 Free PMC article.
References
-
- Antonenko Y. N., Avetisyan A. V., Bakeeva L. E., Chernyak B. V., Chertkov V. A., Domnina L. V., et al. (2008). Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry 73 1273–1287. 10.1134/s0006297908120018 - DOI - PubMed
-
- Arany I., Safirstein R. L. (2003). Cisplatin nephrotoxicity. Semin. Nephrol. 23 460–464. - PubMed
-
- Ascencio-Montiel I. J., Parra E. J., Valladares-Salgado A., Gómez-Zamudio J. H., Kumate-Rodriguez J., Escobedo-de-la-Peña J., et al. (2013). SOD2 gene Val16Ala polymorphism is associated with macroalbuminuria in Mexican type 2 diabetes patients: a comparative study and meta-analysis. BMC Med. Genet. 14:110. 10.1186/1471-2350-14-110 - DOI - PMC - PubMed
-
- Barra D., Schinina M. E., Simmaco M., Bannister J. V., Bannister W. H., Rotilio G., et al. (1984). The primary structure of human liver manganese superoxide dismutase. J. Biol. Chem. 259 12595–12601. - PubMed
-
- Beckman J. S., Koppenol W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271 C1424–C1437. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

