Targeting the Heme Oxygenase 1/Carbon Monoxide Pathway to Resolve Lung Hyper-Inflammation and Restore a Regulated Immune Response in Cystic Fibrosis
- PMID: 32760278
- PMCID: PMC7372134
- DOI: 10.3389/fphar.2020.01059
Targeting the Heme Oxygenase 1/Carbon Monoxide Pathway to Resolve Lung Hyper-Inflammation and Restore a Regulated Immune Response in Cystic Fibrosis
Abstract
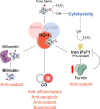
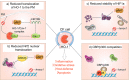
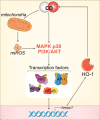
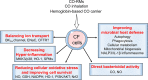
In individuals with cystic fibrosis (CF), lung hyper-inflammation starts early in life and is perpetuated by mucus obstruction and persistent bacterial infections. The continuous tissue damage and scarring caused by non-resolving inflammation leads to bronchiectasis and, ultimately, respiratory failure. Macrophages (MΦs) are key regulators of immune response and host defense. We and others have shown that, in CF, MΦs are hyper-inflammatory and exhibit reduced bactericidal activity. Thus, MΦs contribute to the inability of CF lung tissues to control the inflammatory response or restore tissue homeostasis. The non-resolving hyper-inflammation in CF lungs is attributed to an impairment of several signaling pathways associated with resolution of the inflammatory response, including the heme oxygenase-1/carbon monoxide (HO-1/CO) pathway. HO-1 is an enzyme that degrades heme groups, leading to the production of potent antioxidant, anti-inflammatory, and bactericidal mediators, such as biliverdin, bilirubin, and CO. This pathway is fundamental to re-establishing cellular homeostasis in response to various insults, such as oxidative stress and infection. Monocytes/MΦs rely on abundant induction of the HO-1/CO pathway for a controlled immune response and for potent bactericidal activity. Here, we discuss studies showing that blunted HO-1 activation in CF-affected cells contributes to hyper-inflammation and defective host defense against bacteria. We dissect potential cellular mechanisms that may lead to decreased HO-1 induction in CF cells. We review literature suggesting that induction of HO-1 may be beneficial for the treatment of CF lung disease. Finally, we discuss recent studies highlighting how endogenous HO-1 can be induced by administration of controlled doses of CO to reduce lung hyper-inflammation, oxidative stress, bacterial infection, and dysfunctional ion transport, which are all hallmarks of CF lung disease.
Keywords: CO-releasing molecules; carbon monoxide (CO); cystic fibrosis (CF); heme oxygenase-1 (HO-1); lung inflammation; monocyte/macrophages.
Copyright © 2020 Di Pietro, Öz, Murray and Bruscia.
Figures
Similar articles
-
Recruitment of monocytes primed to express heme oxygenase-1 ameliorates pathological lung inflammation in cystic fibrosis.Exp Mol Med. 2022 May;54(5):639-652. doi: 10.1038/s12276-022-00770-8. Epub 2022 May 17. Exp Mol Med. 2022. PMID: 35581352 Free PMC article.
-
Reduced caveolin-1 promotes hyperinflammation due to abnormal heme oxygenase-1 localization in lipopolysaccharide-challenged macrophages with dysfunctional cystic fibrosis transmembrane conductance regulator.J Immunol. 2013 May 15;190(10):5196-206. doi: 10.4049/jimmunol.1201607. Epub 2013 Apr 19. J Immunol. 2013. PMID: 23606537 Free PMC article.
-
Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders.Antioxidants (Basel). 2022 Mar 15;11(3):555. doi: 10.3390/antiox11030555. Antioxidants (Basel). 2022. PMID: 35326205 Free PMC article. Review.
-
Heme Oxygenase-1 and Its Metabolites Carbon Monoxide and Biliverdin, but Not Iron, Exert Antiviral Activity against Porcine Circovirus Type 3.Microbiol Spectr. 2023 Jun 15;11(3):e0506022. doi: 10.1128/spectrum.05060-22. Epub 2023 May 4. Microbiol Spectr. 2023. PMID: 37140466 Free PMC article.
-
Therapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury.Curr Pharm Des. 2017;23(26):3884-3898. doi: 10.2174/1381612823666170413122439. Curr Pharm Des. 2017. PMID: 28412905 Review.
Cited by
-
The Impact of Highly Effective Modulator Therapy on Cystic Fibrosis Microbiology and Inflammation.Clin Chest Med. 2022 Dec;43(4):647-665. doi: 10.1016/j.ccm.2022.06.007. Clin Chest Med. 2022. PMID: 36344072 Free PMC article. Review.
-
Dual Carbonic Anhydrase IX/XII Inhibitors and Carbon Monoxide Releasing Molecules Modulate LPS-Mediated Inflammation in Mouse Macrophages.Antioxidants (Basel). 2021 Jan 5;10(1):56. doi: 10.3390/antiox10010056. Antioxidants (Basel). 2021. PMID: 33466457 Free PMC article.
-
Protective Effect and Mechanism of Placenta Extract on Liver.Nutrients. 2022 Nov 29;14(23):5071. doi: 10.3390/nu14235071. Nutrients. 2022. PMID: 36501102 Free PMC article. Review.
-
Recruitment of monocytes primed to express heme oxygenase-1 ameliorates pathological lung inflammation in cystic fibrosis.Exp Mol Med. 2022 May;54(5):639-652. doi: 10.1038/s12276-022-00770-8. Epub 2022 May 17. Exp Mol Med. 2022. PMID: 35581352 Free PMC article.
-
Regulation of inflammation by the antioxidant haem oxygenase 1.Nat Rev Immunol. 2021 Jul;21(7):411-425. doi: 10.1038/s41577-020-00491-x. Epub 2021 Jan 29. Nat Rev Immunol. 2021. PMID: 33514947 Review.
References
-
- Abbattiscianni A. C., Favia M., Mancini M. T., Cardone R. A., Guerra L., Monterisi S., et al. (2016). Correctors of mutant CFTR enhance subcortical cAMP-PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization. J. Cell Sci. 129, 1128–1140. 10.1242/jcs.177907 - DOI - PubMed
-
- Abdulrahman B. A., Khweek A. A., Akhter A., Caution K., Kotrange S., Abdelaziz D. H., et al. (2011). Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy 7, 1359–1370. 10.4161/auto.7.11.17660 - DOI - PMC - PubMed
-
- Abu Jawdeh B. G., Woodle E. S., Leino A. D., Brailey P., Tremblay S., Dorst T., et al. (2018). A phase Ib, open-label, single arm study to assess the safety, pharmacokinetics, and impact on humoral sensitization of SANGUINATE infusion in patients with end-stage renal disease. Clin. Transplant. 32. 10.1111/ctr.13155 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

