Gene therapy for alpha 1-antitrypsin deficiency with an oxidant-resistant human alpha 1-antitrypsin
- PMID: 32759494
- PMCID: PMC7455074
- DOI: 10.1172/jci.insight.135951
Gene therapy for alpha 1-antitrypsin deficiency with an oxidant-resistant human alpha 1-antitrypsin
Abstract
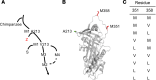
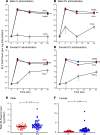
Alpha 1-antitrypsin (AAT) deficiency, a hereditary disorder characterized by low serum levels of functional AAT, is associated with early development of panacinar emphysema. AAT inhibits serine proteases, including neutrophil elastase, protecting the lung from proteolytic destruction. Cigarette smoke, pollution, and inflammatory cell-mediated oxidation of methionine (M) 351 and 358 inactivates AAT, limiting lung protection. In vitro studies using amino acid substitutions demonstrated that replacing M351 with valine (V) and M358 with leucine (L) on a normal M1 alanine (A) 213 background provided maximum antiprotease protection despite oxidant stress. We hypothesized that a onetime administration of a serotype 8 adeno-associated virus (AAV8) gene transfer vector coding for the oxidation-resistant variant AAT (A213/V351/L358; 8/AVL) would maintain antiprotease activity under oxidant stress compared with normal AAT (A213/M351/M358; 8/AMM). 8/AVL was administered via intravenous (IV) and intrapleural (IPL) routes to C57BL/6 mice. High, dose-dependent AAT levels were found in the serum and lung epithelial lining fluid (ELF) of mice administered 8/AVL or 8/AMM by IV or IPL. 8/AVL serum and ELF retained serine protease-inhibitory activity despite oxidant stress while 8/AMM function was abolished. 8/AVL represents a second-generation gene therapy for AAT deficiency providing effective antiprotease protection even with oxidant stress.
Keywords: Gene therapy; Genetic diseases; Pulmonology; Therapeutics.
Conflict of interest statement
Figures



Similar articles
-
Safety of Intravenous Administration of an AAV8 Vector Coding for an Oxidation-Resistant Human α1-Antitrypsin for the Treatment of α1-Antitrypsin Deficiency.Hum Gene Ther. 2023 Feb;34(3-4):139-149. doi: 10.1089/hum.2022.192. Hum Gene Ther. 2023. PMID: 36606685 Free PMC article.
-
Survival Advantage of Both Human Hepatocyte Xenografts and Genome-Edited Hepatocytes for Treatment of α-1 Antitrypsin Deficiency.Mol Ther. 2017 Nov 1;25(11):2477-2489. doi: 10.1016/j.ymthe.2017.09.020. Epub 2017 Sep 25. Mol Ther. 2017. PMID: 29032169 Free PMC article.
-
Intrapleural administration of an AAVrh.10 vector coding for human α1-antitrypsin for the treatment of α1-antitrypsin deficiency.Hum Gene Ther Clin Dev. 2013 Dec;24(4):161-73. doi: 10.1089/humc.2013.168. Hum Gene Ther Clin Dev. 2013. PMID: 24191907
-
Current status of gene therapy for α-1 antitrypsin deficiency.Expert Opin Biol Ther. 2015 Mar;15(3):329-36. doi: 10.1517/14712598.2015.978854. Epub 2014 Nov 3. Expert Opin Biol Ther. 2015. PMID: 25363251 Review.
-
Gene Therapy for Alpha-1 Antitrypsin Deficiency Lung Disease.Ann Am Thorac Soc. 2016 Aug;13 Suppl 4(Suppl 4):S352-69. doi: 10.1513/AnnalsATS.201506-344KV. Ann Am Thorac Soc. 2016. PMID: 27564673 Free PMC article. Review.
Cited by
-
Post-Translational Modifications of Circulating Alpha-1-Antitrypsin Protein.Int J Mol Sci. 2020 Dec 2;21(23):9187. doi: 10.3390/ijms21239187. Int J Mol Sci. 2020. PMID: 33276468 Free PMC article. Review.
-
Alpha-1 Antitrypsin Deficiency.Methods Mol Biol. 2024;2750:1-7. doi: 10.1007/978-1-0716-3605-3_1. Methods Mol Biol. 2024. PMID: 38108962
-
Recent advancements in understanding the genetic involvement of alpha-1 antitrypsin deficiency associated lung disease: a look at future precision medicine approaches.Expert Rev Respir Med. 2022 Feb;16(2):173-182. doi: 10.1080/17476348.2022.2027755. Epub 2022 Jan 13. Expert Rev Respir Med. 2022. PMID: 35025710 Free PMC article. Review.
-
Liver directed adeno-associated viral vectors to treat metabolic disease.J Inherit Metab Dis. 2024 Jan;47(1):22-40. doi: 10.1002/jimd.12637. Epub 2023 Jun 5. J Inherit Metab Dis. 2024. PMID: 37254440 Free PMC article. Review.
-
Protective α1-antitrypsin effects in autoimmune vasculitis are compromised by methionine oxidation.J Clin Invest. 2022 Dec 1;132(23):e160089. doi: 10.1172/JCI160089. J Clin Invest. 2022. PMID: 36125911 Free PMC article.
References
-
- Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84(6A):13–31. - PubMed
-
- de Serres FJ, Blanco I. Prevalence of α1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6(5):277–295. doi: 10.1177/1753465812457113. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

