Necroptosis in Immuno-Oncology and Cancer Immunotherapy
- PMID: 32752206
- PMCID: PMC7464343
- DOI: 10.3390/cells9081823
Necroptosis in Immuno-Oncology and Cancer Immunotherapy
Abstract
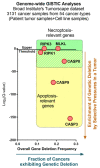
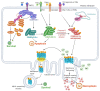
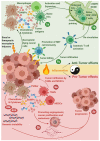
Immune-checkpoint blockers (ICBs) have revolutionized oncology and firmly established the subfield of immuno-oncology. Despite this renaissance, a subset of cancer patients remain unresponsive to ICBs due to widespread immuno-resistance. To "break" cancer cell-driven immuno-resistance, researchers have long floated the idea of therapeutically facilitating the immunogenicity of cancer cells by disrupting tumor-associated immuno-tolerance via conventional anticancer therapies. It is well appreciated that anticancer therapies causing immunogenic or inflammatory cell death are best positioned to productively activate anticancer immunity. A large proportion of studies have emphasized the importance of immunogenic apoptosis (i.e., immunogenic cell death or ICD); yet, it has also emerged that necroptosis, a programmed necrotic cell death pathway, can also be immunogenic. Emergence of a proficient immune profile for necroptosis has important implications for cancer because resistance to apoptosis is one of the major hallmarks of tumors. Putative immunogenic or inflammatory characteristics driven by necroptosis can be of great impact in immuno-oncology. However, as is typical for a highly complex and multi-factorial disease like cancer, a clear cause versus consensus relationship on the immunobiology of necroptosis in cancer cells has been tough to establish. In this review, we discuss the various aspects of necroptosis immunobiology with specific focus on immuno-oncology and cancer immunotherapy.
Keywords: T cells; cytokines; damage-associated molecular patterns (DAMPs); danger signals; dendritic cells; immunogenic cell death; interferons; macrophages; patients; prognostic/predictive biomarkers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity.Nat Rev Cancer. 2024 May;24(5):299-315. doi: 10.1038/s41568-024-00674-x. Epub 2024 Mar 7. Nat Rev Cancer. 2024. PMID: 38454135 Review.
-
Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses.Immunol Rev. 2017 Nov;280(1):126-148. doi: 10.1111/imr.12574. Immunol Rev. 2017. PMID: 29027218 Review.
-
Trial watch: chemotherapy-induced immunogenic cell death in oncology.Oncoimmunology. 2023 Jun 3;12(1):2219591. doi: 10.1080/2162402X.2023.2219591. eCollection 2023. Oncoimmunology. 2023. PMID: 37284695 Free PMC article. Review.
-
Personalized Immuno-Oncology.Med Princ Pract. 2021;30(1):1-16. doi: 10.1159/000511107. Epub 2020 Aug 25. Med Princ Pract. 2021. PMID: 32841942 Free PMC article. Review.
-
Necroptosis-Inducible Polymeric Nanobubbles for Enhanced Cancer Sonoimmunotherapy.Adv Mater. 2020 Apr;32(16):e1907953. doi: 10.1002/adma.201907953. Epub 2020 Mar 3. Adv Mater. 2020. PMID: 32125731
Cited by
-
Development and validation of a necroptosis-related gene prognostic score to predict prognosis and efficiency of immunotherapy in gastric cancer.Front Immunol. 2022 Aug 26;13:977338. doi: 10.3389/fimmu.2022.977338. eCollection 2022. Front Immunol. 2022. PMID: 36159818 Free PMC article.
-
Glioma: bridging the tumor microenvironment, patient immune profiles and novel personalized immunotherapy.Front Immunol. 2024 Jan 11;14:1299064. doi: 10.3389/fimmu.2023.1299064. eCollection 2023. Front Immunol. 2024. PMID: 38274827 Free PMC article. Review.
-
From targeted therapy to a novel way: Immunogenic cell death in lung cancer.Front Med (Lausanne). 2022 Dec 23;9:1102550. doi: 10.3389/fmed.2022.1102550. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36619616 Free PMC article. Review.
-
Prognostic implications of necroptosis-related long noncoding RNA signatures in muscle-invasive bladder cancer.Front Genet. 2022 Dec 2;13:1036098. doi: 10.3389/fgene.2022.1036098. eCollection 2022. Front Genet. 2022. PMID: 36531246 Free PMC article.
-
From (Tool)Bench to Bedside: The Potential of Necroptosis Inhibitors.J Med Chem. 2023 Feb 23;66(4):2361-2385. doi: 10.1021/acs.jmedchem.2c01621. Epub 2023 Feb 13. J Med Chem. 2023. PMID: 36781172 Free PMC article. Review.
References
-
- Gotwals P., Cameron S., Cipolletta D., Cremasco V., Crystal A., Hewes B., Mueller B., Quaratino S., Sabatos-Peyton C., Petruzzelli L., et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. - DOI - PubMed
-
- Chae Y.K., Arya A., Iams W., Cruz M.R., Chandra S., Choi J., Giles F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J. Immunother. Cancer. 2018;6:39. doi: 10.1186/s40425-018-0349-3. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

