PR-DUB maintains the expression of critical genes through FOXK1/2- and ASXL1/2/3-dependent recruitment to chromatin and H2AK119ub1 deubiquitination
- PMID: 32747411
- PMCID: PMC7462075
- DOI: 10.1101/gr.261016.120
PR-DUB maintains the expression of critical genes through FOXK1/2- and ASXL1/2/3-dependent recruitment to chromatin and H2AK119ub1 deubiquitination
Abstract
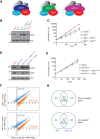
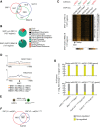
Polycomb group proteins are important for maintaining gene expression patterns and cell identity in metazoans. The mammalian Polycomb repressive deubiquitinase (PR-DUB) complexes catalyze removal of monoubiquitination on lysine 119 of histone H2A (H2AK119ub1) through a multiprotein core comprised of BAP1, HCFC1, FOXK1/2, and OGT in combination with either of ASXL1, 2, or 3. Mutations in PR-DUB components are frequent in cancer. However, mechanistic understanding of PR-DUB function in gene regulation is limited. Here, we show that BAP1 is dependent on the ASXL proteins and FOXK1/2 in facilitating gene activation across the genome. Although PR-DUB was previously shown to cooperate with PRC2, we observed minimal overlap and functional interaction between BAP1 and PRC2 in embryonic stem cells. Collectively, these results demonstrate that PR-DUB, by counteracting accumulation of H2AK119ub1, maintains chromatin in an optimal configuration ensuring expression of genes important for general functions such as cell metabolism and homeostasis.
© 2020 Kolovos et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



Similar articles
-
Polycomb group-mediated histone H2A monoubiquitination in epigenome regulation and nuclear processes.Nat Commun. 2020 Nov 23;11(1):5947. doi: 10.1038/s41467-020-19722-9. Nat Commun. 2020. PMID: 33230107 Free PMC article. Review.
-
BAP1 enhances Polycomb repression by counteracting widespread H2AK119ub1 deposition and chromatin condensation.Mol Cell. 2021 Sep 2;81(17):3526-3541.e8. doi: 10.1016/j.molcel.2021.06.020. Epub 2021 Jun 28. Mol Cell. 2021. PMID: 34186021 Free PMC article.
-
Molecular Regulation of the Polycomb Repressive-Deubiquitinase.Int J Mol Sci. 2020 Oct 22;21(21):7837. doi: 10.3390/ijms21217837. Int J Mol Sci. 2020. PMID: 33105797 Free PMC article. Review.
-
Basis of the H2AK119 specificity of the Polycomb repressive deubiquitinase.Nature. 2023 Apr;616(7955):176-182. doi: 10.1038/s41586-023-05841-y. Epub 2023 Mar 29. Nature. 2023. PMID: 36991118
-
Structural basis of histone H2A lysine 119 deubiquitination by Polycomb repressive deubiquitinase BAP1/ASXL1.Sci Adv. 2023 Aug 9;9(32):eadg9832. doi: 10.1126/sciadv.adg9832. Epub 2023 Aug 9. Sci Adv. 2023. PMID: 37556531 Free PMC article.
Cited by
-
BAP1 in cancer: epigenetic stability and genome integrity.Discov Oncol. 2022 Nov 1;13(1):117. doi: 10.1007/s12672-022-00579-x. Discov Oncol. 2022. PMID: 36318367 Free PMC article. Review.
-
O-GlcNAcylation: the sweet side of epigenetics.Epigenetics Chromatin. 2023 Dec 14;16(1):49. doi: 10.1186/s13072-023-00523-5. Epigenetics Chromatin. 2023. PMID: 38093337 Free PMC article. Review.
-
MBD5 and MBD6 stabilize the BAP1 complex and promote BAP1-dependent cancer.Genome Biol. 2022 Sep 30;23(1):206. doi: 10.1186/s13059-022-02776-x. Genome Biol. 2022. PMID: 36180891 Free PMC article.
-
Polycomb group-mediated histone H2A monoubiquitination in epigenome regulation and nuclear processes.Nat Commun. 2020 Nov 23;11(1):5947. doi: 10.1038/s41467-020-19722-9. Nat Commun. 2020. PMID: 33230107 Free PMC article. Review.
-
Know when to fold 'em: Polycomb complexes in oncogenic 3D genome regulation.Front Cell Dev Biol. 2022 Aug 29;10:986319. doi: 10.3389/fcell.2022.986319. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36105358 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
