A conserved coccidian gene is involved in Toxoplasma sensitivity to the anti-apicomplexan compound, tartrolon E
- PMID: 32738587
- PMCID: PMC7394737
- DOI: 10.1016/j.ijpddr.2020.07.003
A conserved coccidian gene is involved in Toxoplasma sensitivity to the anti-apicomplexan compound, tartrolon E
Abstract
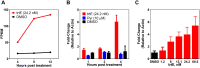
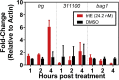
New treatments for the diseases caused by apicomplexans are needed. Recently, we determined that tartrolon E (trtE), a secondary metabolite derived from a shipworm symbiotic bacterium, has broad-spectrum anti-apicomplexan parasite activity. TrtE inhibits apicomplexans at nM concentrations in vitro, including Cryptosporidium parvum, Toxoplasma gondii, Sarcocystis neurona, Plasmodium falciparum, Babesia spp. and Theileria equi. To investigate the mechanism of action of trtE against apicomplexan parasites, we examined changes in the transcriptome of trtE-treated T. gondii parasites. RNA-Seq data revealed that the gene, TGGT1_272370, which is broadly conserved in the coccidia, is significantly upregulated within 4 h of treatment. Using bioinformatics and proteome data available on ToxoDB, we determined that the protein product of this tartrolon E responsive gene (trg) has multiple transmembrane domains, a phosphorylation site, and localizes to the plasma membrane. Deletion of trg in a luciferase-expressing T. gondii strain by CRISPR/Cas9 resulted in a 68% increase in parasite resistance to trtE treatment, supporting a role for the trg protein product in the response of T. gondii to trtE treatment. Trg is conserved in the coccidia, but not in more distantly related apicomplexans, indicating that this response to trtE may be unique to the coccidians, and other mechanisms may be operating in other trtE-sensitive apicomplexans. Uncovering the mechanisms by which trtE inhibits apicomplexans may identify shared pathways critical to apicomplexan parasite survival and advance the search for new treatments.
Keywords: Anti-apicomplexan; CRISPR/Cas9; Drug discovery; Natural products; Tartrolon E; Toxoplasma.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Please declare any financial or personal interests that might be potentially viewed to influence the work presented. Interests could include consultancies, honoraria, patent ownership or other. If there are none state ‘there are none’.
Figures





Similar articles
-
The Marine Compound Tartrolon E Targets the Asexual and Early Sexual Stages of Cryptosporidium parvum.Microorganisms. 2022 Nov 15;10(11):2260. doi: 10.3390/microorganisms10112260. Microorganisms. 2022. PMID: 36422330 Free PMC article.
-
A symbiotic bacterium of shipworms produces a compound with broad spectrum anti-apicomplexan activity.PLoS Pathog. 2020 May 26;16(5):e1008600. doi: 10.1371/journal.ppat.1008600. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32453775 Free PMC article.
-
Evaluation of 4-Amino 2-Anilinoquinazolines against Plasmodium and Other Apicomplexan Parasites In Vitro and in a P. falciparum Humanized NOD-scid IL2Rγnull Mouse Model of Malaria.Antimicrob Agents Chemother. 2019 Feb 26;63(3):e01804-18. doi: 10.1128/AAC.01804-18. Print 2019 Mar. Antimicrob Agents Chemother. 2019. PMID: 30559138 Free PMC article.
-
TKL family kinases in human apicomplexan pathogens.Mol Biochem Parasitol. 2024 Sep;259:111628. doi: 10.1016/j.molbiopara.2024.111628. Epub 2024 May 6. Mol Biochem Parasitol. 2024. PMID: 38719028 Review.
-
Comparative analysis of stage specific gene regulation of apicomplexan parasites: Plasmodium falciparum and Toxoplasma gondii.Infect Disord Drug Targets. 2010 Aug;10(4):303-11. doi: 10.2174/187152610791591593. Infect Disord Drug Targets. 2010. PMID: 20429866 Review.
Cited by
-
Tulathromycin and Diclazuril Lack Efficacy against Theileria haneyi, but Tulathromycin Is Not Associated with Adverse Clinical Effects in Six Treated Adult Horses.Pathogens. 2023 Mar 14;12(3):453. doi: 10.3390/pathogens12030453. Pathogens. 2023. PMID: 36986375 Free PMC article.
-
The Marine Compound Tartrolon E Targets the Asexual and Early Sexual Stages of Cryptosporidium parvum.Microorganisms. 2022 Nov 15;10(11):2260. doi: 10.3390/microorganisms10112260. Microorganisms. 2022. PMID: 36422330 Free PMC article.
-
Development of an Indirect ELISA to Detect Equine Antibodies to Theileria haneyi.Pathogens. 2021 Feb 27;10(3):270. doi: 10.3390/pathogens10030270. Pathogens. 2021. PMID: 33673478 Free PMC article.
-
Imidocarb Dipropionate Lacks Efficacy against Theileria haneyi and Fails to Consistently Clear Theileria equi in Horses Co-Infected with T. haneyi.Pathogens. 2020 Dec 10;9(12):1035. doi: 10.3390/pathogens9121035. Pathogens. 2020. PMID: 33321715 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

