Lactate activation of α-cell KATP channels inhibits glucagon secretion by hyperpolarizing the membrane potential and reducing Ca2+ entry
- PMID: 32736089
- PMCID: PMC7479281
- DOI: 10.1016/j.molmet.2020.101056
Lactate activation of α-cell KATP channels inhibits glucagon secretion by hyperpolarizing the membrane potential and reducing Ca2+ entry
Abstract
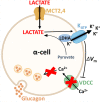
Objective: Elevations in pancreatic α-cell intracellular Ca2+ ([Ca2+]i) lead to glucagon (GCG) secretion. Although glucose inhibits GCG secretion, how lactate and pyruvate control α-cell Ca2+ handling is unknown. Lactate enters cells through monocarboxylate transporters (MCTs) and is also produced during glycolysis by lactate dehydrogenase A (LDHA), an enzyme expressed in α-cells. As lactate activates ATP-sensitive K+ (KATP) channels in cardiomyocytes, lactate may also modulate α-cell KATP. Therefore, this study investigated how lactate signaling controls α-cell Ca2+ handling and GCG secretion.
Methods: Mouse and human islets were used in combination with confocal microscopy, electrophysiology, GCG immunoassays, and fluorescent thallium flux assays to assess α-cell Ca2+ handling, Vm, KATP currents, and GCG secretion.
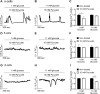
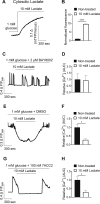
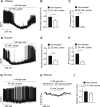
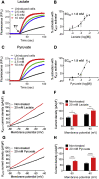
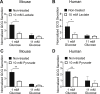
Results: Lactate-inhibited mouse (75 ± 25%) and human (47 ± 9%) α-cell [Ca2+]i fluctuations only under low-glucose conditions (1 mM) but had no effect on β- or δ-cells [Ca2+]i. Glyburide inhibition of KATP channels restored α-cell [Ca2+]i fluctuations in the presence of lactate. Lactate transport into α-cells via MCTs hyperpolarized mouse (14 ± 1 mV) and human (12 ± 1 mV) α-cell Vm and activated KATP channels. Interestingly, pyruvate showed a similar KATP activation profile and α-cell [Ca2+]i inhibition as lactate. Lactate-induced inhibition of α-cell [Ca2+]i influx resulted in reduced GCG secretion in mouse (62 ± 6%) and human (43 ± 13%) islets.
Conclusions: These data demonstrate for the first time that lactate entry into α-cells through MCTs results in KATP activation, Vm hyperpolarization, reduced [Ca2+]i, and inhibition of GCG secretion. Thus, taken together, these data indicate that lactate either within α-cells and/or elevated in serum could serve as important modulators of α-cell function.
Keywords: Ca(2+) handling; Glucagon secretion; K(ATP) channels; Lactate; Pyruvate; α-cells.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures


Similar articles
-
Glucose-mediated inhibition of calcium-activated potassium channels limits α-cell calcium influx and glucagon secretion.Am J Physiol Endocrinol Metab. 2019 Apr 1;316(4):E646-E659. doi: 10.1152/ajpendo.00342.2018. Epub 2019 Jan 29. Am J Physiol Endocrinol Metab. 2019. PMID: 30694690 Free PMC article.
-
KATP channel blockers control glucagon secretion by distinct mechanisms: A direct stimulation of α-cells involving a [Ca2+]c rise and an indirect inhibition mediated by somatostatin.Mol Metab. 2021 Nov;53:101268. doi: 10.1016/j.molmet.2021.101268. Epub 2021 Jun 9. Mol Metab. 2021. PMID: 34118477 Free PMC article.
-
Glucose inhibits glucagon secretion by decreasing [Ca2+]c and by reducing the efficacy of Ca2+ on exocytosis via somatostatin-dependent and independent mechanisms.Mol Metab. 2022 Jul;61:101495. doi: 10.1016/j.molmet.2022.101495. Epub 2022 Apr 11. Mol Metab. 2022. PMID: 35421610 Free PMC article.
-
'Resistance is futile?' - paradoxical inhibitory effects of KATP channel closure in glucagon-secreting α-cells.J Physiol. 2020 Nov;598(21):4765-4780. doi: 10.1113/JP279775. Epub 2020 Aug 7. J Physiol. 2020. PMID: 32716554 Free PMC article. Review.
-
Regulation of glucagon secretion by glucose: paracrine, intrinsic or both?Diabetes Obes Metab. 2011 Oct;13 Suppl 1:95-105. doi: 10.1111/j.1463-1326.2011.01450.x. Diabetes Obes Metab. 2011. PMID: 21824262 Review.
Cited by
-
Leucine Suppresses α-Cell cAMP and Glucagon Secretion via a Combination of Cell-Intrinsic and Islet Paracrine Signaling.Diabetes. 2024 Sep 1;73(9):1426-1439. doi: 10.2337/db23-1013. Diabetes. 2024. PMID: 38870025
-
ROCK1 regulates insulin secretion from β-cells.Mol Metab. 2022 Dec;66:101625. doi: 10.1016/j.molmet.2022.101625. Epub 2022 Oct 29. Mol Metab. 2022. PMID: 36374631 Free PMC article.
-
SLC17A1/3 transporters mediate renal excretion of Lac-Phe in mice and humans.Nat Commun. 2024 Aug 12;15(1):6895. doi: 10.1038/s41467-024-51174-3. Nat Commun. 2024. PMID: 39134528 Free PMC article.
-
Liraglutide increases islet Ca2+ oscillation frequency and insulin secretion by activating hyperpolarization-activated cyclic nucleotide-gated channels.Diabetes Obes Metab. 2022 Sep;24(9):1741-1752. doi: 10.1111/dom.14747. Epub 2022 May 30. Diabetes Obes Metab. 2022. PMID: 35546791 Free PMC article.
-
α-cell electrophysiology and the regulation of glucagon secretion.J Endocrinol. 2023 Jun 26;258(2):e220295. doi: 10.1530/JOE-22-0295. Print 2023 Aug 1. J Endocrinol. 2023. PMID: 37159865 Free PMC article. Review.
References
-
- Miller R.A., Birnbaum M.J. Glucagon: acute actions on hepatic metabolism. Diabetologia. 2016;59(7):1376–1381. - PubMed
-
- Gerich J.E., Frankel B.J., Fanska R., West L., Forsham P.H., Grodsky G.M. Calcium dependency of glucagon secretion from the in vitro perfused rat pancreas. Endocrinology. 1974;94(5):1381–1385. - PubMed
-
- Lundquist I., Fanska R., Grodsky G.M. Interaction of calcium and glucose on glucagon secretion. Endocrinology. 1976;99(5):1304–1312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

