STAT3 is a key molecule in the oncogenic behavior of diffuse intrinsic pontine glioma
- PMID: 32724445
- PMCID: PMC7377111
- DOI: 10.3892/ol.2020.11699
STAT3 is a key molecule in the oncogenic behavior of diffuse intrinsic pontine glioma
Abstract
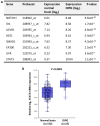
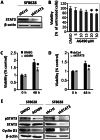
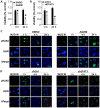
Diffuse intrinsic pontine glioma (DIPG) is one of the most lethal childhood brain tumors. This tumor is unique because it is detected exclusively in the ventral pons of patients aged between 6 and 7 years, which suggests a developmental nature of its formation. Signal transducer and activator of transcription 3 (STAT3) is a critical molecule for the differentiation of neural stem cells into astrocytes during neurodevelopment. Additionally, STAT3 is associated with oncogenesis and the epithelial-mesenchymal transition (EMT) in various types of tumor. In recent years, several studies have demonstrated the oncogenic role of STAT3 in high-grade gliomas. However, the role of STAT3 in DIPG at the cellular level remains unknown. To assess the possible association between gliogenesis and DIPG, the expression levels of various molecules participating in the differentiation of neural stem cells were compared between normal brain control tissues and DIPG tissues using open public data. All of the screened genes exhibited significantly increased expression in DIPG tissues compared with normal tissues. As STAT3 expression was the most increased, the effect of STAT3 inhibition in a DIPG cell line was assessed via STAT3 short hairpin (sh)RNA transfection and treatment with AG490, a STAT3 inhibitor. Changes in viability, apoptosis, EMT and radiation therapy efficiency were also evaluated. Downregulation of STAT3 resulted in decreased cyclin D1 expression and cell viability, migration and invasion. Additionally, treatment with STAT3 shRNA or AG490 suppressed the EMT phenotype. Finally, when radiation was administered in combination with STAT3 inhibition, the therapeutic efficiency, assessed by cell viability and DNA damage repair, was increased. The present results suggest that STAT3 is a potential therapeutic target in DIPG, especially when combined with radiation therapy.
Keywords: astrogliogenesis; diffuse intrinsic pontine glioma; epithelial-mesenchymal transition; signal transducer and activator of transcription 3.
Copyright © 2020, Spandidos Publications.
Figures

Similar articles
-
Polo-like Kinase 1 as a potential therapeutic target in Diffuse Intrinsic Pontine Glioma.BMC Cancer. 2016 Aug 18;16:647. doi: 10.1186/s12885-016-2690-6. BMC Cancer. 2016. PMID: 27538997 Free PMC article.
-
OLIG2 maintenance is not essential for diffuse intrinsic pontine glioma cell line growth but regulates tumor phenotypes.Neuro Oncol. 2021 Jul 1;23(7):1183-1196. doi: 10.1093/neuonc/noab016. Neuro Oncol. 2021. PMID: 33539525 Free PMC article.
-
Preclinical evaluation of convection-enhanced delivery of liposomal doxorubicin to treat pediatric diffuse intrinsic pontine glioma and thalamic high-grade glioma.J Neurosurg Pediatr. 2017 May;19(5):518-530. doi: 10.3171/2016.9.PEDS16152. Epub 2017 Feb 17. J Neurosurg Pediatr. 2017. PMID: 28291423
-
Signaling pathways and mesenchymal transition in pediatric high-grade glioma.Cell Mol Life Sci. 2018 Mar;75(5):871-887. doi: 10.1007/s00018-017-2714-7. Epub 2017 Nov 21. Cell Mol Life Sci. 2018. PMID: 29164272 Free PMC article. Review.
-
Signal Transduction in Diffuse Intrinsic Pontine Glioma.Proteomics. 2019 Nov;19(21-22):e1800479. doi: 10.1002/pmic.201800479. Epub 2019 Aug 14. Proteomics. 2019. PMID: 31328874 Review.
Cited by
-
Impact of phospholipase C β1 in glioblastoma: a study on the main mechanisms of tumor aggressiveness.Cell Mol Life Sci. 2022 Mar 18;79(4):195. doi: 10.1007/s00018-022-04198-1. Cell Mol Life Sci. 2022. PMID: 35303162 Free PMC article.
-
Advanced Pediatric Diffuse Pontine Glioma Murine Models Pave the Way towards Precision Medicine.Cancers (Basel). 2021 Mar 5;13(5):1114. doi: 10.3390/cancers13051114. Cancers (Basel). 2021. PMID: 33807733 Free PMC article. Review.
-
Radiotherapy and radio-sensitization in H3K27M -mutated diffuse midline gliomas.CNS Neurosci Ther. 2023 Jul;29(7):1721-1737. doi: 10.1111/cns.14225. Epub 2023 May 8. CNS Neurosci Ther. 2023. PMID: 37157237 Free PMC article. Review.
-
Therapeutic Targets in Diffuse Midline Gliomas-An Emerging Landscape.Cancers (Basel). 2021 Dec 13;13(24):6251. doi: 10.3390/cancers13246251. Cancers (Basel). 2021. PMID: 34944870 Free PMC article. Review.
-
Pediatric Diffuse Midline Gliomas: An Unfinished Puzzle.Diagnostics (Basel). 2022 Aug 25;12(9):2064. doi: 10.3390/diagnostics12092064. Diagnostics (Basel). 2022. PMID: 36140466 Free PMC article. Review.
References
-
- Pollack IF, Jakacki RI, Blaney SM, Hancock ML, Kieran MW, Phillips P, Kun LE, Friedman H, Packer R, Banerjee A, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: A pediatric brain tumor consortium report. Neuro Oncol. 2007;9:145–160. doi: 10.1215/15228517-2006-031. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
