MKP-1 overexpression is associated with chemoresistance in bladder cancer via the MAPK pathway
- PMID: 32724417
- PMCID: PMC7377201
- DOI: 10.3892/ol.2020.11741
MKP-1 overexpression is associated with chemoresistance in bladder cancer via the MAPK pathway
Abstract
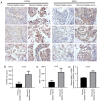
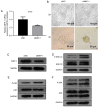
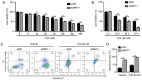
Mitogen activated protein kinase phosphatase-1 (MKP-1) has been revealed to be overexpressed in bladder cancer, particularly in non-muscle invasive bladder cancer. MKP-1 may also be associated with chemotherapy resistance. However, the underlying mechanism is yet to be elucidated. The current study investigated the expression of MKP-1 by performing immunohistochemistry in surgically resected specimens obtained from primary and recurrent patients with bladder cancer. The results revealed that MKP-1 expression increased in recurrent patients. Additionally, a 3D model of the human bladder cancer cell line, RT112, was established to determine the role of MKP-1 in drug resistance. The results demonstrated that MKP-1 overexpression protected bladder cancer cells against cell death. Contrarily, MKP-1 knockdown was revealed to sensitize cells to death. In addition, the application of MAPK inhibitors effectively increased RT112 cell sensitivity to pirarubicin. In conclusion, the results of the current study indicated that MKP-1 treatment resulted in bladder cancer cell chemoresistance via JNK, ERK and p38 pathways. MKP-1 may also serve as a potential therapeutic target for chemoresistance in patients with bladder cancer.
Keywords: 3D model; RT112 cell; bladder cancer; fibroblast growth factor 3; mitogen activated protein kinase phosphatase-1; resistance.
Copyright: © Lei et al.
Figures

Similar articles
-
Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients.Clin Cancer Res. 2004 Jun 1;10(11):3639-49. doi: 10.1158/1078-0432.CCR-03-0771. Clin Cancer Res. 2004. PMID: 15173070
-
Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance.Cancer Res. 2007 May 1;67(9):4459-66. doi: 10.1158/0008-5472.CAN-06-2644. Cancer Res. 2007. PMID: 17483361
-
Mitogen-activated protein kinase phosphatase-1 in human breast cancer independently predicts prognosis and is repressed by doxorubicin.Clin Cancer Res. 2009 May 15;15(10):3530-9. doi: 10.1158/1078-0432.CCR-08-2070. Epub 2009 May 5. Clin Cancer Res. 2009. PMID: 19417026
-
Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis.Lab Invest. 1997 Jan;76(1):37-51. Lab Invest. 1997. PMID: 9010448
-
Targeting mitogen-activated protein kinase phosphatase-1 (MKP-1): structure-based design of MKP-1 inhibitors and upregulators.Curr Med Chem. 2012;19(2):163-73. doi: 10.2174/092986712803414196. Curr Med Chem. 2012. PMID: 22320295 Review.
Cited by
-
A Novel CpG Methylation Risk Indicator for Predicting Prognosis in Bladder Cancer.Front Cell Dev Biol. 2021 Sep 1;9:642650. doi: 10.3389/fcell.2021.642650. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34540821 Free PMC article.
-
Construction and verification of a novel hypoxia-related lncRNA signature related with survival outcomes and immune microenvironment of bladder urothelial carcinoma by weighted gene co-expression network analysis.Front Genet. 2022 Aug 31;13:952369. doi: 10.3389/fgene.2022.952369. eCollection 2022. Front Genet. 2022. PMID: 36118856 Free PMC article.
-
Sulforaphane Impact on Reactive Oxygen Species (ROS) in Bladder Carcinoma.Int J Mol Sci. 2021 May 31;22(11):5938. doi: 10.3390/ijms22115938. Int J Mol Sci. 2021. PMID: 34073079 Free PMC article. Review.
-
MiR-205-3p suppresses bladder cancer progression via GLO1 mediated P38/ERK activation.BMC Cancer. 2023 Oct 9;23(1):956. doi: 10.1186/s12885-023-11175-9. BMC Cancer. 2023. PMID: 37814205 Free PMC article.
-
IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer.Biomed Res Int. 2021 Apr 23;2021:5535578. doi: 10.1155/2021/5535578. eCollection 2021. Biomed Res Int. 2021. PMID: 33981768 Free PMC article.
References
-
- Liu S, Hou J, Zhang H, Wu Y, Hu M, Zhang L, Xu J, Na R, Jiang H, Ding Q. The evaluation of the risk factors for non-muscle invasive bladder cancer (NMIBC) recurrence after transurethral resection (TURBt) in Chinese population. PLoS One. 2014;10:e0123617. doi: 10.1371/journal.pone.0123617. - DOI - PMC - PubMed
-
- Miki T, Nonomura N, Kojima Y, Okuyama A, Nakano E, Kiyohara H, Fujioka H, Koide T, Wakatsuki A, Kuroda H, et al. A randomized study on intravesical pirarubicin (THP) chemoprophylaxis of recurrence after transurethral resection of superficial bladder cancer. Hinyokika Kiyo. 1997;43:907–912. (In Japanese) - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
