Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates
- PMID: 32722908
- PMCID: PMC7449230
- DOI: 10.1056/NEJMoa2024671
Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates
Abstract
Background: Vaccines to prevent coronavirus disease 2019 (Covid-19) are urgently needed. The effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines on viral replication in both upper and lower airways is important to evaluate in nonhuman primates.
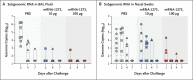
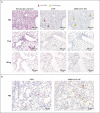
Methods: Nonhuman primates received 10 or 100 μg of mRNA-1273, a vaccine encoding the prefusion-stabilized spike protein of SARS-CoV-2, or no vaccine. Antibody and T-cell responses were assessed before upper- and lower-airway challenge with SARS-CoV-2. Active viral replication and viral genomes in bronchoalveolar-lavage (BAL) fluid and nasal swab specimens were assessed by polymerase chain reaction, and histopathological analysis and viral quantification were performed on lung-tissue specimens.
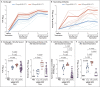
Results: The mRNA-1273 vaccine candidate induced antibody levels exceeding those in human convalescent-phase serum, with live-virus reciprocal 50% inhibitory dilution (ID50) geometric mean titers of 501 in the 10-μg dose group and 3481 in the 100-μg dose group. Vaccination induced type 1 helper T-cell (Th1)-biased CD4 T-cell responses and low or undetectable Th2 or CD8 T-cell responses. Viral replication was not detectable in BAL fluid by day 2 after challenge in seven of eight animals in both vaccinated groups. No viral replication was detectable in the nose of any of the eight animals in the 100-μg dose group by day 2 after challenge, and limited inflammation or detectable viral genome or antigen was noted in lungs of animals in either vaccine group.
Conclusions: Vaccination of nonhuman primates with mRNA-1273 induced robust SARS-CoV-2 neutralizing activity, rapid protection in the upper and lower airways, and no pathologic changes in the lung. (Funded by the National Institutes of Health and others.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Similar articles
-
DNA vaccine protection against SARS-CoV-2 in rhesus macaques.Science. 2020 Aug 14;369(6505):806-811. doi: 10.1126/science.abc6284. Epub 2020 May 20. Science. 2020. PMID: 32434945 Free PMC article.
-
ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques.Nature. 2020 Oct;586(7830):578-582. doi: 10.1038/s41586-020-2608-y. Epub 2020 Jul 30. Nature. 2020. PMID: 32731258 Free PMC article.
-
A measles-vectored vaccine candidate expressing prefusion-stabilized SARS-CoV-2 spike protein brought to phase I/II clinical trials: protection of African green monkeys from COVID-19 disease.J Virol. 2024 May 14;98(5):e0176223. doi: 10.1128/jvi.01762-23. Epub 2024 Apr 2. J Virol. 2024. PMID: 38563762 Free PMC article.
-
COVID-19 Vaccine: A comprehensive status report.Virus Res. 2020 Oct 15;288:198114. doi: 10.1016/j.virusres.2020.198114. Epub 2020 Aug 13. Virus Res. 2020. PMID: 32800805 Free PMC article. Review.
-
Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies.Nat Microbiol. 2020 Oct;5(10):1185-1191. doi: 10.1038/s41564-020-00789-5. Epub 2020 Sep 9. Nat Microbiol. 2020. PMID: 32908214 Review.
Cited by
-
Review of Covid-19 vaccine clinical trials - A puzzle with missing pieces.Int J Biol Sci. 2021 Apr 10;17(6):1461-1468. doi: 10.7150/ijbs.59170. eCollection 2021. Int J Biol Sci. 2021. PMID: 33907509 Free PMC article. Review.
-
Interim analysis of an open-label randomized controlled trial evaluating nasal irrigations in non-hospitalized patients with coronavirus disease 2019.Int Forum Allergy Rhinol. 2020 Dec;10(12):1325-1328. doi: 10.1002/alr.22703. Epub 2020 Oct 20. Int Forum Allergy Rhinol. 2020. PMID: 32914928 Free PMC article. Clinical Trial. No abstract available.
-
The Food Anti-Microbials β-Phenylethylamine (-HCl) and Ethyl Acetoacetate Do Not Change during the Heating Process.Antibiotics (Basel). 2021 Apr 10;10(4):418. doi: 10.3390/antibiotics10040418. Antibiotics (Basel). 2021. PMID: 33920266 Free PMC article.
-
Harnessing preexisting influenza virus-specific immunity increases antibody responses against SARS-CoV-2.J Virol. 2024 Feb 20;98(2):e0157123. doi: 10.1128/jvi.01571-23. Epub 2024 Jan 11. J Virol. 2024. PMID: 38206036 Free PMC article.
-
Lipid-nanoparticle-encapsulated mRNA vaccines induce protective memory CD8 T cells against a lethal viral infection.Mol Ther. 2021 Sep 1;29(9):2769-2781. doi: 10.1016/j.ymthe.2021.05.011. Epub 2021 May 14. Mol Ther. 2021. PMID: 33992803 Free PMC article.
References
-
- Callaway E, Cyranoski D, Mallapaty S, Stoye E, Tollefson J. The coronavirus pandemic in five powerful charts. Nature 2020;579:482-483. - PubMed
-
- Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. July 1, 2020. (https://www.medrxiv.org/content/10.1101/2020.06.30.20142570v1). preprint.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
