Cancer and HIV-1 Infection: Patterns of Chronic Antigen Exposure
- PMID: 32714330
- PMCID: PMC7344140
- DOI: 10.3389/fimmu.2020.01350
Cancer and HIV-1 Infection: Patterns of Chronic Antigen Exposure
Abstract
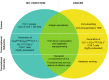
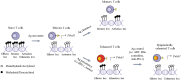
The main role of the human immune system is to eliminate cells presenting foreign antigens and abnormal patterns, while maintaining self-tolerance. However, when facing highly variable pathogens or antigens very similar to self-antigens, this system can fail in completely eliminating the anomalies, leading to the establishment of chronic pathologies. Prototypical examples of immune system defeat are cancer and Human Immunodeficiency Virus-1 (HIV-1) infection. In both conditions, the immune system is persistently exposed to antigens leading to systemic inflammation, lack of generation of long-term memory and exhaustion of effector cells. This triggers a negative feedback loop where effector cells are unable to resolve the pathology and cannot be replaced due to the lack of a pool of undifferentiated, self-renewing memory T cells. In addition, in an attempt to reduce tissue damage due to chronic inflammation, antigen presenting cells and myeloid components of the immune system activate systemic regulatory and tolerogenic programs. Beside these homologies shared between cancer and HIV-1 infection, the immune system can be shaped differently depending on the type and distribution of the eliciting antigens with ultimate consequences at the phenotypic and functional level of immune exhaustion. T cell differentiation, functionality, cytotoxic potential and proliferation reserve, immune-cell polarization, upregulation of negative regulators (immune checkpoint molecules) are indeed directly linked to the quantitative and qualitative differences in priming and recalling conditions. Better understanding of distinct mechanisms and functional consequences underlying disease-specific immune cell dysfunction will contribute to further improve and personalize immunotherapy. In the present review, we describe relevant players of immune cell exhaustion in cancer and HIV-1 infection, and enumerate the best-defined hallmarks of T cell dysfunction. Moreover, we highlight shared and divergent aspects of T cell exhaustion and T cell activation to the best of current knowledge.
Keywords: HIV infection; anergy; cancer; cellular immunity; exhaustion; immune checkpoint; lymphocytes; senescence.
Copyright © 2020 Vigano, Bobisse, Coukos, Perreau and Harari.
Figures
Similar articles
-
T cell exhaustion in cancer: Mechanisms and clinical implications.J Cell Biochem. 2018 Jun;119(6):4279-4286. doi: 10.1002/jcb.26645. Epub 2018 Mar 7. J Cell Biochem. 2018. PMID: 29274296 Review.
-
Anergy and human immunodeficiency virus infection.Med Hypotheses. 2001 Mar;56(3):376-80. doi: 10.1054/mehy.2000.1220. Med Hypotheses. 2001. PMID: 11359364
-
Tolerance and exhaustion: defining mechanisms of T cell dysfunction.Trends Immunol. 2014 Feb;35(2):51-60. doi: 10.1016/j.it.2013.10.001. Epub 2013 Nov 6. Trends Immunol. 2014. PMID: 24210163 Free PMC article. Review.
-
Exhaustion of T lymphocytes in the tumor microenvironment: Significance and effective mechanisms.Cell Immunol. 2017 Dec;322:1-14. doi: 10.1016/j.cellimm.2017.10.002. Epub 2017 Oct 10. Cell Immunol. 2017. PMID: 29079339 Review.
-
T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment.Curr Opin Immunol. 2013 Apr;25(2):214-21. doi: 10.1016/j.coi.2012.12.003. Epub 2013 Jan 6. Curr Opin Immunol. 2013. PMID: 23298609 Free PMC article. Review.
Cited by
-
Interferon regulatory factor 4 deficiency in CD8+ T cells abrogates terminal effector differentiation and promotes transplant acceptance.Immunology. 2020 Dec;161(4):364-379. doi: 10.1111/imm.13258. Epub 2020 Oct 12. Immunology. 2020. PMID: 32892353 Free PMC article.
-
Molecular, metabolic, and functional CD4 T cell paralysis in the lymph node impedes tumor control.Cell Rep. 2023 Sep 26;42(9):113047. doi: 10.1016/j.celrep.2023.113047. Epub 2023 Aug 30. Cell Rep. 2023. PMID: 37651234 Free PMC article.
-
Identification of CD8+ T cell subsets that normalize in early-treated people living with HIV receiving antiretroviral therapy.AIDS Res Ther. 2022 Sep 14;19(1):42. doi: 10.1186/s12981-022-00465-0. AIDS Res Ther. 2022. PMID: 36104716 Free PMC article.
-
Immune mobilising T cell receptors redirect polyclonal CD8+ T cells in chronic HIV infection to form immunological synapses.Sci Rep. 2022 Nov 1;12(1):18366. doi: 10.1038/s41598-022-23228-3. Sci Rep. 2022. PMID: 36319836 Free PMC article.
-
HIV and prior exposure to antiretroviral therapy alter tumour composition and tumour: T-cell associations in diffuse large B-cell lymphoma.Br J Haematol. 2024 Jul;205(1):194-206. doi: 10.1111/bjh.19531. Epub 2024 May 20. Br J Haematol. 2024. PMID: 38769021
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

