Pancreatic Macrophages: Critical Players in Obesity-Promoted Pancreatic Cancer
- PMID: 32709161
- PMCID: PMC7409049
- DOI: 10.3390/cancers12071946
Pancreatic Macrophages: Critical Players in Obesity-Promoted Pancreatic Cancer
Abstract
Obesity is a known risk factor for the development of pancreatic cancer, one of the deadliest types of malignancies. In recent years it has become clear that the pancreatic microenvironment is critically involved and a contributing factor in accelerating pancreatic neoplasia. In this context obesity-associated chronic inflammation plays an important role. Among several immune cells, macrophages have been shown to contribute to obesity-induced tissue inflammation. This review article summarizes the current knowledge about the role of pancreatic macrophages in early pancreatic cancer development. It describes the heterogenous origin and mixture of pancreatic macrophages, their role in pancreatic endocrine and exocrine pathology, and the impact of obesity on islet and stromal macrophages. A model is postulated, by which during obesity monocytes are recruited into the pancreas, where they are polarized into pro-inflammatory macrophages that drive early pancreatic neoplasia. This occurs in the presence of local inflammatory, metabolic, and endocrine signals. A stronger appreciation and more detailed knowledge about the role of macrophages in early pancreatic cancer development will lead to innovative preventive or interceptive strategies.
Keywords: acinar-to-ductal metaplasia; inflammation; macrophages; obesity; pancreatic cancer.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
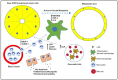
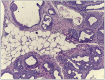
Similar articles
-
Beta cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo.Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4419-24. doi: 10.1073/pnas.0605248104. Epub 2007 Mar 7. Proc Natl Acad Sci U S A. 2007. PMID: 17360539 Free PMC article.
-
Functional significance of macrophages in pancreatic cancer biology.Tumour Biol. 2015 Dec;36(12):9119-26. doi: 10.1007/s13277-015-4127-2. Epub 2015 Sep 28. Tumour Biol. 2015. PMID: 26411672 Free PMC article. Review.
-
Developmental aspects of early pancreatic cancer.Cancer J. 2001 Jul-Aug;7(4):242-50. Cancer J. 2001. PMID: 11561600 Review.
-
NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas.Gastroenterology. 2015 May;148(5):1024-1034.e9. doi: 10.1053/j.gastro.2015.01.033. Epub 2015 Jan 23. Gastroenterology. 2015. PMID: 25623042 Free PMC article.
-
Pancreatic steatosis: an update.Curr Opin Gastroenterol. 2019 Sep;35(5):440-447. doi: 10.1097/MOG.0000000000000566. Curr Opin Gastroenterol. 2019. PMID: 31343416 Review.
Cited by
-
Acinar-to-Ductal Metaplasia (ADM): On the Road to Pancreatic Intraepithelial Neoplasia (PanIN) and Pancreatic Cancer.Int J Mol Sci. 2023 Jun 9;24(12):9946. doi: 10.3390/ijms24129946. Int J Mol Sci. 2023. PMID: 37373094 Free PMC article. Review.
-
A high Diabetes Risk Reduction Score (DRRS) is associated with a better cardio-metabolic profile among obese individuals.BMC Endocr Disord. 2023 Feb 3;23(1):31. doi: 10.1186/s12902-023-01279-5. BMC Endocr Disord. 2023. PMID: 36737726 Free PMC article.
-
Roles of differently polarized macrophages in the initiation and progressionof pancreatic cancer.Front Immunol. 2023 Aug 11;14:1237711. doi: 10.3389/fimmu.2023.1237711. eCollection 2023. Front Immunol. 2023. PMID: 37638028 Free PMC article. Review.
-
Obesity and Pancreatic Cancer: Insight into Mechanisms.Cancers (Basel). 2021 Oct 10;13(20):5067. doi: 10.3390/cancers13205067. Cancers (Basel). 2021. PMID: 34680216 Free PMC article. Review.
-
Macrophage-induced reactive oxygen species in the initiation of pancreatic cancer: a mini-review.Front Immunol. 2024 Mar 21;15:1278807. doi: 10.3389/fimmu.2024.1278807. eCollection 2024. Front Immunol. 2024. PMID: 38576613 Free PMC article. Review.

