Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer's Disease: Cell Cycle Reentry and Beyond
- PMID: 32708313
- PMCID: PMC7409121
- DOI: 10.3390/cells9071746
Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer's Disease: Cell Cycle Reentry and Beyond
Abstract
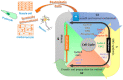
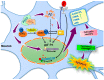
Inhibitor of DNA-binding/differentiation (Id) proteins, a family of helix-loop-helix (HLH) proteins that includes four members of Id1 to Id4 in mammalian cells, are critical for regulating cell growth, differentiation, senescence, cell cycle progression, and increasing angiogenesis and vasculogenesis, as well as accelerating the ability of cell migration. Alzheimer's disease (AD), the most common neurodegenerative disease in the adult population, manifests the signs of cognitive decline, behavioral changes, and functional impairment. The underlying mechanisms for AD are not well-clarified yet, but the aggregation of amyloid-beta peptides (Aβs), the major components in the senile plaques observed in AD brains, contributes significantly to the disease progression. Emerging evidence reveals that aberrant cell cycle reentry may play a central role in Aβ-induced neuronal demise. Recently, we have shown that several signaling mediators, including Id1, hypoxia-inducible factor-1 (HIF-1), cyclin-dependent kinases-5 (CDK5), and sonic hedgehog (Shh), may contribute to Aβ-induced cell cycle reentry in postmitotic neurons; furthermore, Id1 and CDK5/p25 mutually antagonize the expression/activity of each other. Therefore, Id proteins may potentially have clinical applications in AD. In this review article, we introduce the underlying mechanisms for cell cycle dysregulation in AD and present some examples, including our own studies, to show different aspects of Id1 in terms of cell cycle reentry and other signaling that may be crucial to alter the neuronal fates in this devastating neurodegenerative disease. A thorough understanding of the underlying mechanisms may provide a rationale to make an earlier intervention before the occurrence of cell cycle reentry and subsequent apoptosis in the fully differentiated neurons during the progression of AD or other neurodegenerative diseases.
Keywords: Alzheimer’s disease; cell cycle reentry; inhibitor of DNA-binding/differentiation proteins; neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Roles of Id1/HIF-1 and CDK5/HIF-1 in cell cycle reentry induced by amyloid-beta peptide in post-mitotic cortical neuron.Biochim Biophys Acta Mol Cell Res. 2020 Apr;1867(4):118628. doi: 10.1016/j.bbamcr.2019.118628. Epub 2019 Dec 26. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 31884068
-
Id1 and Sonic Hedgehog Mediate Cell Cycle Reentry and Apoptosis Induced by Amyloid Beta-Peptide in Post-mitotic Cortical Neurons.Mol Neurobiol. 2019 Jan;56(1):465-489. doi: 10.1007/s12035-018-1098-5. Epub 2018 May 2. Mol Neurobiol. 2019. PMID: 29721855
-
Inhibitor of Differentiation-1 and Hypoxia-Inducible Factor-1 Mediate Sonic Hedgehog Induction by Amyloid Beta-Peptide in Rat Cortical Neurons.Mol Neurobiol. 2016 Mar;53(2):793-809. doi: 10.1007/s12035-014-9046-5. Epub 2014 Dec 15. Mol Neurobiol. 2016. PMID: 25502463
-
The Role of Cdk5 in Alzheimer's Disease.Mol Neurobiol. 2016 Sep;53(7):4328-42. doi: 10.1007/s12035-015-9369-x. Epub 2015 Jul 31. Mol Neurobiol. 2016. PMID: 26227906 Review.
-
Aneuploidy, chromosomal missegregation, and cell cycle reentry in Alzheimer's disease.Acta Neurobiol Exp (Wars). 2009;69(2):232-53. doi: 10.55782/ane-2009-1748. Acta Neurobiol Exp (Wars). 2009. PMID: 19593337 Review.
Cited by
-
Transcriptional Profiles of Cell Fate Transitions Reveal Early Drivers of Neuronal Apoptosis and Survival.Cells. 2021 Nov 19;10(11):3238. doi: 10.3390/cells10113238. Cells. 2021. PMID: 34831459 Free PMC article.
-
Role of cyclin-dependent kinase 5 in early brain injury following experimental subarachnoid hemorrhage.Exp Ther Med. 2022 Feb;23(2):147. doi: 10.3892/etm.2021.11070. Epub 2021 Dec 15. Exp Ther Med. 2022. PMID: 35069828 Free PMC article.
-
Ameliorating Alzheimer's-like Pathology by Minocycline via Inhibiting Cdk5/p25 Signaling.Curr Neuropharmacol. 2022 Aug 3;20(9):1783-1792. doi: 10.2174/1570159X19666211202124925. Curr Neuropharmacol. 2022. PMID: 34856907 Free PMC article.
-
Predicting the influence of Circ_0059706 expression on prognosis in patients with acute myeloid leukemia using classical statistics and machine learning.Front Genet. 2022 Oct 21;13:961142. doi: 10.3389/fgene.2022.961142. eCollection 2022. Front Genet. 2022. PMID: 36338954 Free PMC article.
-
An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception.Diversity (Basel). 2021 Aug;13(8):364. doi: 10.3390/d13080364. Epub 2021 Aug 7. Diversity (Basel). 2021. PMID: 35505776 Free PMC article.
References
-
- Kawamoto E.M., Lepsch L.B., Boaventura M.F., Munhoz C.D., Lima L.S., Yshii L.M., Avellar M.C., Curi R. Amyloid beta-peptide activates nuclear factor-kappaB through an N-methyl-D-aspartate signaling pathway in cultured cerebellar cells. J. Neurosci. Res. 2008;86:845–860. doi: 10.1002/jnr.21548. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

