The Lipid Receptor G2A (GPR132) Mediates Macrophage Migration in Nerve Injury-Induced Neuropathic Pain
- PMID: 32708184
- PMCID: PMC7409160
- DOI: 10.3390/cells9071740
The Lipid Receptor G2A (GPR132) Mediates Macrophage Migration in Nerve Injury-Induced Neuropathic Pain
Abstract
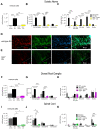
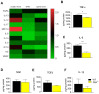
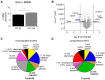
Nerve injury-induced neuropathic pain is difficult to treat and mechanistically characterized by strong neuroimmune interactions, involving signaling lipids that act via specific G-protein coupled receptors. Here, we investigated the role of the signaling lipid receptor G2A (GPR132) in nerve injury-induced neuropathic pain using the robust spared nerve injury (SNI) mouse model. We found that the concentrations of the G2A agonist 9-HODE (9-Hydroxyoctadecadienoic acid) are strongly increased at the site of nerve injury during neuropathic pain. Moreover, G2A-deficient mice show a strong reduction of mechanical hypersensitivity after nerve injury. This phenotype is accompanied by a massive reduction of invading macrophages and neutrophils in G2A-deficient mice and a strongly reduced release of the proalgesic mediators TNFα, IL-6 and VEGF at the site of injury. Using a global proteome analysis to identify the underlying signaling pathways, we found that G2A activation in macrophages initiates MyD88-PI3K-AKT signaling and transient MMP9 release to trigger cytoskeleton remodeling and migration. We conclude that G2A-deficiency reduces inflammatory responses by decreasing the number of immune cells and the release of proinflammatory cytokines and growth factors at the site of nerve injury. Inhibiting the G2A receptor after nerve injury may reduce immune cell-mediated peripheral sensitization and may thus ameliorate neuropathic pain.
Keywords: 9-HODE; G2A; GPR132; macrophage migration; neuropathic pain; oxidized linoleic acid metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The G2A receptor (GPR132) contributes to oxaliplatin-induced mechanical pain hypersensitivity.Sci Rep. 2017 Mar 27;7(1):446. doi: 10.1038/s41598-017-00591-0. Sci Rep. 2017. PMID: 28348394 Free PMC article.
-
The G2A Receptor Controls Polarization of Macrophage by Determining Their Localization Within the Inflamed Tissue.Front Immunol. 2018 Oct 1;9:2261. doi: 10.3389/fimmu.2018.02261. eCollection 2018. Front Immunol. 2018. PMID: 30327654 Free PMC article.
-
Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain.Mol Pain. 2018 Jan-Dec;14:1744806918764979. doi: 10.1177/1744806918764979. Mol Pain. 2018. PMID: 29546785 Free PMC article.
-
G2A as a receptor for oxidized free fatty acids.Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):66-72. doi: 10.1016/j.prostaglandins.2008.11.002. Epub 2008 Nov 21. Prostaglandins Other Lipid Mediat. 2009. PMID: 19063986 Review.
-
Pharmacological Regulation of Neuropathic Pain Driven by Inflammatory Macrophages.Int J Mol Sci. 2017 Nov 1;18(11):2296. doi: 10.3390/ijms18112296. Int J Mol Sci. 2017. PMID: 29104252 Free PMC article. Review.
Cited by
-
Loss of the mammalian G-protein coupled receptor, G2A, modulates severity of invasive pulmonary aspergillosis.Front Immunol. 2023 Jun 26;14:1173544. doi: 10.3389/fimmu.2023.1173544. eCollection 2023. Front Immunol. 2023. PMID: 37435068 Free PMC article.
-
Activation of orphan receptor GPR132 induces cell differentiation in acute myeloid leukemia.Cell Death Dis. 2022 Nov 27;13(11):1004. doi: 10.1038/s41419-022-05434-z. Cell Death Dis. 2022. PMID: 36437247 Free PMC article.
-
The role of orphan G protein-coupled receptors in pain.Heliyon. 2024 Mar 26;10(7):e28818. doi: 10.1016/j.heliyon.2024.e28818. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38590871 Free PMC article. Review.
-
Endogenous Derivatives of Linoleic Acid and their Stable Analogs Are Potential Pain Mediators.JID Innov. 2022 Dec 26;3(2):100177. doi: 10.1016/j.xjidi.2022.100177. eCollection 2023 Mar. JID Innov. 2022. PMID: 36876220 Free PMC article.
-
Proton-Sensing GPCRs in Health and Disease.Cells. 2021 Aug 10;10(8):2050. doi: 10.3390/cells10082050. Cells. 2021. PMID: 34440817 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

