Lack of antibody-mediated cross-protection between SARS-CoV-2 and SARS-CoV infections
- PMID: 32707445
- PMCID: PMC7372296
- DOI: 10.1016/j.ebiom.2020.102890
Lack of antibody-mediated cross-protection between SARS-CoV-2 and SARS-CoV infections
Abstract
Background: The novel coronavirus (SARS-CoV-2) shares approximately 80% whole genome sequence identity and 66% spike (S) protein identity with that of SARS-CoV. The cross-neutralization between these viruses is currently not well-defined.
Methods: Here, by using the live SARS-CoV-2 virus infection assay as well as HIV-1 based pseudotyped-virus carrying the spike (S) gene of the SARS-CoV-2 (ppSARS-2) and SARS-CoV (ppSARS), we examined whether infections with SARS-CoV and SARS-CoV-2 can induce cross-neutralizing antibodies.
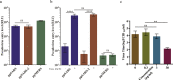
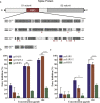
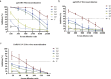
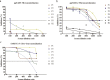
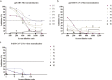
Findings: We confirmed that SARS-CoV-2 infects cells via angiotensin converting enzyme 2 (ACE2), the functional receptor for SARS-CoV, and we also found that the recombinant receptor binding domain (RBD) of the S protein of SARS-CoV effectively inhibits ppSARS-2 entry in Huh7.5 cells. However, convalescent sera from SARS-CoV and SARS-CoV-2 patients showed high neutralizing activity only against the homologous virus, with no or limited cross-neutralization activity against the other pseudotyped virus. Similar results were also observed in vaccination studies in mice.
Interpretation: Our study demonstrates that although both SARS-CoV and SARS-CoV-2 use ACE2 as a cellular receptor, the neutralization epitopes are not shared by these two closely-related viruses, highlighting challenges towards developing a universal vaccine against SARS-CoV related viruses.
Funding: This work was supported by the National Key Research and Development Program of China, the National Major Project for Control and Prevention of Infectious Disease in China, and the One Belt and One Road Major Project for infectious diseases.
Keywords: ACE2; COVID-19; CoV; Cross-neutralization; SARS-CoV-2.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interests with respect to this study.
Figures
Similar articles
-
Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies.Cell Mol Immunol. 2020 Jun;17(6):621-630. doi: 10.1038/s41423-020-0458-z. Epub 2020 May 15. Cell Mol Immunol. 2020. PMID: 32415260 Free PMC article.
-
Identification of SARS-CoV RBD-targeting monoclonal antibodies with cross-reactive or neutralizing activity against SARS-CoV-2.Antiviral Res. 2020 Jul;179:104820. doi: 10.1016/j.antiviral.2020.104820. Epub 2020 May 13. Antiviral Res. 2020. PMID: 32405117 Free PMC article.
-
Characterization of MW06, a human monoclonal antibody with cross-neutralization activity against both SARS-CoV-2 and SARS-CoV.MAbs. 2021 Jan-Dec;13(1):1953683. doi: 10.1080/19420862.2021.1953683. MAbs. 2021. PMID: 34313527 Free PMC article.
-
Protective Immunity against SARS Subunit Vaccine Candidates Based on Spike Protein: Lessons for Coronavirus Vaccine Development.J Immunol Res. 2020 Jul 18;2020:7201752. doi: 10.1155/2020/7201752. eCollection 2020. J Immunol Res. 2020. PMID: 32695833 Free PMC article. Review.
-
Potent human monoclonal antibodies against SARS CoV, Nipah and Hendra viruses.Expert Opin Biol Ther. 2009 Mar;9(3):355-68. doi: 10.1517/14712590902763755. Expert Opin Biol Ther. 2009. PMID: 19216624 Free PMC article. Review.
Cited by
-
Preexisting antibodies targeting SARS-CoV-2 S2 cross-react with commensal gut bacteria and impact COVID-19 vaccine induced immunity.Gut Microbes. 2022 Jan-Dec;14(1):2117503. doi: 10.1080/19490976.2022.2117503. Gut Microbes. 2022. PMID: 36100957 Free PMC article.
-
Inactivated SARS-CoV-2 Vaccine Shows Cross-Protection against Bat SARS-Related Coronaviruses in Human ACE2 Transgenic Mice.J Virol. 2022 Apr 27;96(8):e0016922. doi: 10.1128/jvi.00169-22. Epub 2022 Mar 28. J Virol. 2022. PMID: 35343762 Free PMC article.
-
Challenges and developments in universal vaccine design against SARS-CoV-2 variants.NPJ Vaccines. 2022 Dec 19;7(1):167. doi: 10.1038/s41541-022-00597-4. NPJ Vaccines. 2022. PMID: 36535982 Free PMC article. Review.
-
Cross-Reactivity of Human, Wild Boar, and Farm Animal Sera from Pre- and Post-Pandemic Periods with Alpha- and Βeta-Coronaviruses (CoV), including SARS-CoV-2.Viruses. 2023 Dec 23;16(1):34. doi: 10.3390/v16010034. Viruses. 2023. PMID: 38257734 Free PMC article.
-
How immunity from and interaction with seasonal coronaviruses can shape SARS-CoV-2 epidemiology.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2108395118. doi: 10.1073/pnas.2108395118. Proc Natl Acad Sci U S A. 2021. PMID: 34873059 Free PMC article.
References
-
- World Health Organization. COVID-19. 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

