The Current State of Molecular Testing in the BRAF-Mutated Melanoma Landscape
- PMID: 32695793
- PMCID: PMC7338720
- DOI: 10.3389/fmolb.2020.00113
The Current State of Molecular Testing in the BRAF-Mutated Melanoma Landscape
Abstract
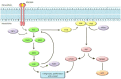
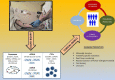
The incidence of melanoma, among the most lethal cancers, is widespread and increasing. Metastatic melanoma has a poor prognosis, representing about 90% of skin cancer mortality. The increased knowledge of tumor biology and the greater understanding of the immune system role in the anti-tumor response has allowed us to develop a more rational approach to systemic therapies. The discovery of activating BRAF mutations in half of all melanomas has led to the development of molecularly targeted therapy with BRAF and MEK inhibitors, which dramatically improved outcomes of patients with stage IV BRAF-mutant melanoma. More recently, the results of clinical phase III studies conducted in the adjuvant setting led to the combined administration of BRAF and MEK inhibitors also in patients with resected high-risk melanoma (stage III). Therefore, BRAF mutation testing has become a priority to determine the oncologist's choice and course of therapy. In this review, we will report the molecular biology-based strategies used for BRAF mutation detection with the main advantages and disadvantages of the most commonly used diagnostic strategies. The timing of such molecular assessment in patients with cutaneous melanoma will be discussed, and we will also examine considerations and approaches for accurate and effective BRAF testing.
Keywords: BRAF mutation; NGS; liquid biopsy; melanoma; molecular techniques; targeted therapy.
Copyright © 2020 Vanni, Tanda, Spagnolo, Andreotti, Bruno and Ghiorzo.
Figures
Similar articles
-
BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies.Onco Targets Ther. 2015 Jan 16;8:157-68. doi: 10.2147/OTT.S39096. eCollection 2015. Onco Targets Ther. 2015. PMID: 25653539 Free PMC article. Review.
-
BRAF, NRAS and C-KIT Advanced Melanoma: Clinico-pathological Features, Targeted-Therapy Strategies and Survival.Anticancer Res. 2017 Dec;37(12):7043-7048. doi: 10.21873/anticanres.12175. Anticancer Res. 2017. PMID: 29187493
-
Frontline approach to metastatic BRAF-mutant melanoma diagnosis, molecular evaluation, and treatment choice.Am Soc Clin Oncol Educ Book. 2014:e412-21. doi: 10.14694/EdBook_AM.2014.34.e412. Am Soc Clin Oncol Educ Book. 2014. PMID: 24857132 Review.
-
Current Advances in the Treatment of BRAF-Mutant Melanoma.Cancers (Basel). 2020 Feb 19;12(2):482. doi: 10.3390/cancers12020482. Cancers (Basel). 2020. PMID: 32092958 Free PMC article. Review.
-
Treatment of BRAF-mutant melanoma: the role of vemurafenib and other therapies.Clin Pharmacol Ther. 2014 Jan;95(1):24-31. doi: 10.1038/clpt.2013.197. Epub 2013 Sep 30. Clin Pharmacol Ther. 2014. PMID: 24080641 Review.
Cited by
-
Oligoribonucleotide interference-PCR-based methods for the sensitive and accurate detection of KRAS mutations.Biol Methods Protoc. 2024 Oct 1;9(1):bpae071. doi: 10.1093/biomethods/bpae071. eCollection 2024. Biol Methods Protoc. 2024. PMID: 39478810 Free PMC article.
-
Non-BRAF Mutant Melanoma: Molecular Features and Therapeutical Implications.Front Mol Biosci. 2020 Jul 24;7:172. doi: 10.3389/fmolb.2020.00172. eCollection 2020. Front Mol Biosci. 2020. PMID: 32850962 Free PMC article. Review.
-
BRAF V600E Mutation in Malignant Melanoma-A Romanian Research Experience.Medicina (Kaunas). 2024 Feb 20;60(3):351. doi: 10.3390/medicina60030351. Medicina (Kaunas). 2024. PMID: 38541077 Free PMC article.
-
Integrating circulating-free DNA (cfDNA) analysis into clinical practice: opportunities and challenges.Br J Cancer. 2022 Sep;127(4):592-602. doi: 10.1038/s41416-022-01776-9. Epub 2022 Mar 26. Br J Cancer. 2022. PMID: 35347327 Free PMC article. Review.
-
Oncogenic BRAF and p53 Interplay in Melanoma Cells and the Effects of the HDAC Inhibitor ITF2357 (Givinostat).Int J Mol Sci. 2023 May 23;24(11):9148. doi: 10.3390/ijms24119148. Int J Mol Sci. 2023. PMID: 37298104 Free PMC article.
References
-
- Anderson S., Bloom K. J., Vallera D. U., Rueschoff J., Meldrum C., Schilling R., et al. . (2012). Multisite analytic performance studies of a real-time polymerase chain reaction assay for the detection of BRAF V600E mutations in formalin-fixed, paraffin-embedded tissue specimens of malignant melanoma. Arch. Pathol. Lab. Med. 136, 1385–1391. 10.5858/arpa.2011-0505-OA - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials