Glucose-dependent partitioning of arginine to the urea cycle protects β-cells from inflammation
- PMID: 32694660
- PMCID: PMC7568475
- DOI: 10.1038/s42255-020-0199-4
Glucose-dependent partitioning of arginine to the urea cycle protects β-cells from inflammation
Abstract
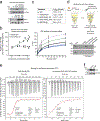
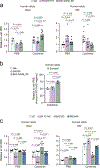
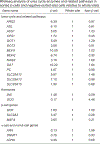
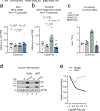
Chronic inflammation is linked to diverse disease processes, but the intrinsic mechanisms that determine cellular sensitivity to inflammation are incompletely understood. Here, we show the contribution of glucose metabolism to inflammation-induced changes in the survival of pancreatic islet β-cells. Using metabolomic, biochemical and functional analyses, we investigate the protective versus non-protective effects of glucose in the presence of pro-inflammatory cytokines. When protective, glucose metabolism augments anaplerotic input into the TCA cycle via pyruvate carboxylase (PC) activity, leading to increased aspartate levels. This metabolic mechanism supports the argininosuccinate shunt, which fuels ureagenesis from arginine and conversely diminishes arginine utilization for production of nitric oxide (NO), a chief mediator of inflammatory cytotoxicity. Activation of the PC-urea cycle axis is sufficient to suppress NO synthesis and shield cells from death in the context of inflammation and other stress paradigms. Overall, these studies uncover a previously unappreciated link between glucose metabolism and arginine-utilizing pathways via PC-directed ureagenesis as a protective mechanism.
Conflict of interest statement
Competing interests
The authors declare the following competing interests: J.A.M. serves on the SAB of 908 Devices, and has received sponsored research support from AstraZeneca and Vertex. L.D.W. is a scientific co-founder and shareholder in Aileron Therapeutics. R.G.J. is a scientific advisory board member for Immunomet Therapeutics and consultant for Agios Pharmaceuticals.
Figures





Similar articles
-
Pyruvate-Carboxylase-Mediated Anaplerosis Promotes Antioxidant Capacity by Sustaining TCA Cycle and Redox Metabolism in Liver.Cell Metab. 2019 Jun 4;29(6):1291-1305.e8. doi: 10.1016/j.cmet.2019.03.014. Epub 2019 Apr 18. Cell Metab. 2019. PMID: 31006591 Free PMC article.
-
L-arginine is essential for pancreatic β-cell functional integrity, metabolism and defense from inflammatory challenge.J Endocrinol. 2011 Oct;211(1):87-97. doi: 10.1530/JOE-11-0236. Epub 2011 Jul 22. J Endocrinol. 2011. PMID: 21784771
-
Toxicity generated through inhibition of pyruvate carboxylase and carnitine palmitoyl transferase-1 is similar to high glucose/palmitate-induced glucolipotoxicity in INS-1 beta cells.Mol Cell Endocrinol. 2014 Mar 5;383(1-2):48-59. doi: 10.1016/j.mce.2013.12.002. Epub 2013 Dec 11. Mol Cell Endocrinol. 2014. PMID: 24333689
-
Argininosuccinate lyase deficiency-argininosuccinic aciduria and beyond.Am J Med Genet C Semin Med Genet. 2011 Feb 15;157C(1):45-53. doi: 10.1002/ajmg.c.30289. Epub 2011 Feb 10. Am J Med Genet C Semin Med Genet. 2011. PMID: 21312326 Free PMC article. Review.
-
The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: Going round in circles?Islets. 2011 Nov-Dec;3(6):302-19. doi: 10.4161/isl.3.6.17806. Epub 2011 Nov 1. Islets. 2011. PMID: 21934355 Free PMC article. Review.
Cited by
-
Arginase: Biological and Therapeutic Implications in Diabetes Mellitus and Its Complications.Oxid Med Cell Longev. 2022 Oct 26;2022:2419412. doi: 10.1155/2022/2419412. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36338341 Free PMC article. Review.
-
Mitochondrial bioenergetics, metabolism, and beyond in pancreatic β-cells and diabetes.Front Mol Biosci. 2024 Feb 9;11:1354199. doi: 10.3389/fmolb.2024.1354199. eCollection 2024. Front Mol Biosci. 2024. PMID: 38404962 Free PMC article. Review.
-
Small molecule metabolites: discovery of biomarkers and therapeutic targets.Signal Transduct Target Ther. 2023 Mar 20;8(1):132. doi: 10.1038/s41392-023-01399-3. Signal Transduct Target Ther. 2023. PMID: 36941259 Free PMC article. Review.
-
Changes in Mitochondria-Related Gene Expression upon Acupuncture at LR3 in the D-Galactosamine-Induced Liver Damage Rat Model.Evid Based Complement Alternat Med. 2022 Jun 29;2022:3294273. doi: 10.1155/2022/3294273. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35928244 Free PMC article.
-
Pithecellobium clypearia: Amelioration Effect on Imiquimod-Induced Psoriasis in Mice Based on a Tissue Metabonomic Analysis.Front Pharmacol. 2021 Sep 17;12:748772. doi: 10.3389/fphar.2021.748772. eCollection 2021. Front Pharmacol. 2021. PMID: 34603060 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

