Peptide vaccination directed against IDO1-expressing immune cells elicits CD8+ and CD4+ T-cell-mediated antitumor immunity and enhanced anti-PD1 responses
- PMID: 32690770
- PMCID: PMC7373332
- DOI: 10.1136/jitc-2020-000605
Peptide vaccination directed against IDO1-expressing immune cells elicits CD8+ and CD4+ T-cell-mediated antitumor immunity and enhanced anti-PD1 responses
Abstract
Background: The tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase 1 (IDO1), which subverts T-cell immunity at multiple levels, is itself subject to inherent T-cell reactivity. This intriguing deviation from central tolerance has been interpreted as counterbalancing IDO1-mediated immunosuppression. Based on this hypothesis, clinical studies employing an IDO1 peptide-based vaccine approach for cancer treatment have been initiated, but there remains a pressing need to further investigate the immunological ramifications of stimulating the anti-IDO1 T-cell response in this manner.
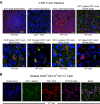
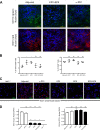
Methods: CT26 colon carcinoma tumors were evaluated for expression of IDO1 protein by western blot analysis, immunofluorescence microscopy and flow cytometry. Mouse IDO1-derived peptides, predicted to bind either major histocompatibility complex (MHC) class I or II of the H2d BALB/c strain, were emulsified in 50% Montanide for prophylactic or therapeutic vaccine treatment of CT26 tumor-bearing mice initiated either 7 days prior to or following tumor cell injection, respectively. In some therapeutic treatment experiments, administration of programmed cell death protein 1-binding antibody (anti-PD1 antibody) or epacadostat was concurrently initiated. Tumor size was determined by caliper measurements and comparative tumor growth suppression was assessed by longitudinal analyses of tumor growth data. For adoptive transfer, T cells from complete responder animals were isolated using paramagnetic beads and fluorescence-activated cell sorting.
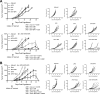
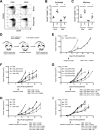
Results: This study identifies mouse MHC class I-directed and II-directed, IDO1-derived peptides capable of eliciting antitumor responses, despite finding IDO1 expressed exclusively in tumor-infiltrating immune cells. Treatment of established tumors with anti-PD1 antibody and class I-directed but not class II-directed IDO1 peptide vaccines produced an enhanced antitumor response. Likewise, class I-directed and II-directed IDO1 peptides elicited an enhanced combinatorial response, suggesting distinct mechanisms of action. Consistent with this interpretation, adoptive transfer of isolated CD8+ T cells from class I and CD4+ T cells from class II peptide-vaccinated responder mice delayed tumor growth. The class II-directed response was completely IDO1-dependent while the class I-directed response included an IDO1-independent component consistent with antigen spread.
Conclusions: The in vivo antitumor effects demonstrated with IDO1-based vaccines via targeting of the tumor microenvironment highlight the utility of mouse models for further exploration and refinement of this novel vaccine-based approach to IDO1-directed cancer therapy and its potential to improve patient response rates to anti-PD1 therapy.
Keywords: adaptive immunity; immunotherapy; indoleamine-pyrrole 2,3,-dioxygenase; programmed cell death 1 receptor; vaccination.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: IO Biotech, which provided financial support for this project, is conducting IDO1 peptide vaccine-based clinical trials. KLK, IL and AWP are employed by IO Biotech, M-BZ is a Founder, Chief Executive Officer and a shareholder in IO Biotech, MHA is a Founder, Chief Scientific Officer and shareholder in IO Biotech and AJM is a Scientific Advisory Board member and shareholder in IO Biotech.
Figures


Similar articles
-
Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response.Front Immunol. 2019 Aug 14;10:1939. doi: 10.3389/fimmu.2019.01939. eCollection 2019. Front Immunol. 2019. PMID: 31475002 Free PMC article.
-
Combination vaccine based on citrullinated vimentin and enolase peptides induces potent CD4-mediated anti-tumor responses.J Immunother Cancer. 2020 Jun;8(1):e000560. doi: 10.1136/jitc-2020-000560. J Immunother Cancer. 2020. PMID: 32561639 Free PMC article.
-
miR-448 targets IDO1 and regulates CD8+ T cell response in human colon cancer.J Immunother Cancer. 2019 Aug 7;7(1):210. doi: 10.1186/s40425-019-0691-0. J Immunother Cancer. 2019. PMID: 31391111 Free PMC article.
-
Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond.Semin Immunopathol. 2019 Jan;41(1):41-48. doi: 10.1007/s00281-018-0702-0. Epub 2018 Sep 10. Semin Immunopathol. 2019. PMID: 30203227 Review.
-
CIITA-Driven MHC Class II Expressing Tumor Cells as Antigen Presenting Cell Performers: Toward the Construction of an Optimal Anti-tumor Vaccine.Front Immunol. 2019 Jul 30;10:1806. doi: 10.3389/fimmu.2019.01806. eCollection 2019. Front Immunol. 2019. PMID: 31417570 Free PMC article. Review.
Cited by
-
Arginase-1 specific CD8+ T cells react toward malignant and regulatory myeloid cells.Oncoimmunology. 2024 Feb 22;13(1):2318053. doi: 10.1080/2162402X.2024.2318053. eCollection 2024. Oncoimmunology. 2024. PMID: 38404966 Free PMC article.
-
Long-term follow-up of anti-PD-1 naïve patients with metastatic melanoma treated with IDO/PD-L1 targeting peptide vaccine and nivolumab.J Immunother Cancer. 2023 May;11(5):e006755. doi: 10.1136/jitc-2023-006755. J Immunother Cancer. 2023. PMID: 37217243 Free PMC article.
-
Arginase-2-specific cytotoxic T cells specifically recognize functional regulatory T cells.J Immunother Cancer. 2022 Oct;10(10):e005326. doi: 10.1136/jitc-2022-005326. J Immunother Cancer. 2022. PMID: 36316062 Free PMC article.
-
Recent Advances and Challenges in Cancer Immunotherapy.Cancers (Basel). 2022 Aug 17;14(16):3972. doi: 10.3390/cancers14163972. Cancers (Basel). 2022. PMID: 36010965 Free PMC article. Review.
-
A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma.Nat Med. 2021 Dec;27(12):2212-2223. doi: 10.1038/s41591-021-01544-x. Epub 2021 Dec 9. Nat Med. 2021. PMID: 34887574 Free PMC article. Clinical Trial.
References
-
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. with a report of ten original cases. 1893. Clin Orthop Relat Res 1991;105:487–511. - PubMed
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
