An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates
- PMID: 32690628
- PMCID: PMC7402629
- DOI: 10.1126/scitranslmed.abc9396
An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates
Abstract
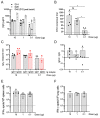
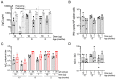
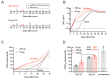
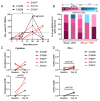
The coronavirus disease 2019 (COVID-19) pandemic, caused by infection with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is having a deleterious impact on health services and the global economy, highlighting the urgent need for an effective vaccine. Such a vaccine would need to rapidly confer protection after one or two doses and would need to be manufactured using components suitable for scale up. Here, we developed an Alphavirus-derived replicon RNA vaccine candidate, repRNA-CoV2S, encoding the SARS-CoV-2 spike (S) protein. The RNA replicons were formulated with lipid inorganic nanoparticles (LIONs) that were designed to enhance vaccine stability, delivery, and immunogenicity. We show that a single intramuscular injection of the LION/repRNA-CoV2S vaccine in mice elicited robust production of anti-SARS-CoV-2 S protein IgG antibody isotypes indicative of a type 1 T helper cell response. A prime/boost regimen induced potent T cell responses in mice including antigen-specific responses in the lung and spleen. Prime-only immunization of aged (17 months old) mice induced smaller immune responses compared to young mice, but this difference was abrogated by booster immunization. In nonhuman primates, prime-only immunization in one intramuscular injection site or prime/boost immunizations in five intramuscular injection sites elicited modest T cell responses and robust antibody responses. The antibody responses persisted for at least 70 days and neutralized SARS-CoV-2 at titers comparable to those in human serum samples collected from individuals convalescing from COVID-19. These data support further development of LION/repRNA-CoV2S as a vaccine candidate for prophylactic protection against SARS-CoV-2 infection.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures

Similar articles
-
Single-dose replicating RNA vaccine induces neutralizing antibodies against SARS-CoV-2 in nonhuman primates.bioRxiv [Preprint]. 2020 May 28:2020.05.28.121640. doi: 10.1101/2020.05.28.121640. bioRxiv. 2020. PMID: 32511417 Free PMC article. Preprint.
-
Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates.N Engl J Med. 2020 Oct 15;383(16):1544-1555. doi: 10.1056/NEJMoa2024671. Epub 2020 Jul 28. N Engl J Med. 2020. PMID: 32722908 Free PMC article.
-
A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity.Nature. 2020 Oct;586(7830):572-577. doi: 10.1038/s41586-020-2599-8. Epub 2020 Jul 29. Nature. 2020. PMID: 32726802
-
Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses.Int Immunopharmacol. 2020 Sep;86:106717. doi: 10.1016/j.intimp.2020.106717. Epub 2020 Jun 18. Int Immunopharmacol. 2020. PMID: 32585611 Free PMC article. Review.
-
SARS-CoV-2: A New Song Recalls an Old Melody.Cell Host Microbe. 2020 May 13;27(5):692-694. doi: 10.1016/j.chom.2020.04.019. Cell Host Microbe. 2020. PMID: 32407706 Free PMC article. Review.
Cited by
-
Antibodies targeting the Crimean-Congo Hemorrhagic Fever Virus nucleoprotein protect via TRIM21.Nat Commun. 2024 Oct 25;15(1):9236. doi: 10.1038/s41467-024-53362-7. Nat Commun. 2024. PMID: 39455551 Free PMC article.
-
mRNA vaccines for infectious diseases - advances, challenges and opportunities.Nat Rev Drug Discov. 2024 Nov;23(11):838-861. doi: 10.1038/s41573-024-01042-y. Epub 2024 Oct 4. Nat Rev Drug Discov. 2024. PMID: 39367276 Review.
-
mRNA vaccines: Past, present, future.Asian J Pharm Sci. 2022 Jul;17(4):491-522. doi: 10.1016/j.ajps.2022.05.003. Epub 2022 Jun 30. Asian J Pharm Sci. 2022. PMID: 36105317 Free PMC article. Review.
-
Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2.NPJ Vaccines. 2021 May 13;6(1):74. doi: 10.1038/s41541-021-00336-1. NPJ Vaccines. 2021. PMID: 33986272 Free PMC article. Review.
-
A Phase I/II Clinical Trial of Intradermal, Controllable Self-Replicating Ribonucleic Acid Vaccine EXG-5003 against SARS-CoV-2.Vaccines (Basel). 2023 Nov 27;11(12):1767. doi: 10.3390/vaccines11121767. Vaccines (Basel). 2023. PMID: 38140172 Free PMC article.
References
-
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E. C., Zhang Y.-Z., A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020). 10.1038/s41586-020-2008-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

