Nivolumab plus Ipilimumab versus Existing Immunotherapies in Patients with PD-L1-Positive Advanced Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis
- PMID: 32679702
- PMCID: PMC7409193
- DOI: 10.3390/cancers12071905
Nivolumab plus Ipilimumab versus Existing Immunotherapies in Patients with PD-L1-Positive Advanced Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis
Abstract
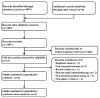
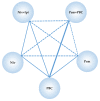
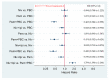
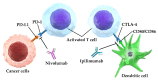
No head-to-head trials have compared the efficacy and safety of nivolumab (Niv) plus ipilimumab (Ipi) combination therapy (Niv+Ipi) and existing regimens with immunotherapies approved as first-line treatment in patients with programmed cell death ligand 1 (PD-L1)-positive previously untreated advanced non-small cell lung cancer (NSCLC). We conducted a network meta-analysis of four relevant Phase Ⅲ trials to compare the efficacy and safety of Niv+Ipi, pembrolizumab (Pem) plus platinum-based chemotherapy (PBC) (Pem+PBC), Pem, Niv, or PBC using Bayesian analysis. The primary efficacy endpoint was progression-free survival (PFS) in patients with advanced NSCLC with PD-L1 expression ≥1%. The primary safety endpoint was the incidence of Grade 3-5 drug-related adverse events (G3-5AEs). Efficacy and safety were ranked using surface under the cumulative ranking curve (SUCRA). With regard to PFS, Niv+Ipi was inferior to Pem+PBC, and superior to Pem, Niv, or PBC alone. SUCRA ranking showed Pem+PBC had the highest efficacy for PFS, followed by Niv+Ipi, Niv, PBC, and Pem. The safety outcome analysis revealed Niv+Ipi was generally well tolerated compared to existing immunotherapy regimens. These results provide clinical information regarding the efficacy and safety of Niv+Ipi and indicate the possibility of the Niv+Ipi combination as a new therapeutic option in PD-L1-positive advanced NSCLC.
Keywords: indirect treatment comparison; ipilimumab; network meta-analysis; nivolumab; non-small cell lung cancer; overall survival; pembrolizumab; programmed cell death ligand 1; progression-free survival; systematic review.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without PD-L1 selection: A systematic review and network meta-analysis.Chin Med J (Engl). 2023 Sep 20;136(18):2156-2165. doi: 10.1097/CM9.0000000000002750. Epub 2023 Aug 18. Chin Med J (Engl). 2023. PMID: 37596898 Free PMC article.
-
Long-term comparative efficacy and safety of nivolumab plus ipilimumab relative to other first-line therapies for advanced non-small-cell lung cancer: A systematic literature review and network meta-analysis.Lung Cancer. 2023 Mar;177:11-20. doi: 10.1016/j.lungcan.2023.01.006. Epub 2023 Jan 11. Lung Cancer. 2023. PMID: 36669321
-
Feasibility and safety of PD-1/L1 inhibitors for non-small cell lung cancer in front-line treatment: a Bayesian network meta-analysis.Transl Lung Cancer Res. 2020 Apr;9(2):188-203. doi: 10.21037/tlcr.2020.02.14. Transl Lung Cancer Res. 2020. PMID: 32420059 Free PMC article.
-
A Network Meta-Analysis of the Differences in Effectiveness and Safety between Nivolumab and Targeted Drug Therapy in Metastatic Renal Cell Carcinoma.J Oncol. 2022 Mar 28;2022:5805289. doi: 10.1155/2022/5805289. eCollection 2022. J Oncol. 2022. PMID: 35386213 Free PMC article. Review.
-
Comparative Efficacy and Safety of Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors for Refractory or Relapsed Advanced Non-Small-Cell Lung Cancer-A Systematic Review and Network Meta-Analysis.Cancers (Basel). 2020 Dec 27;13(1):52. doi: 10.3390/cancers13010052. Cancers (Basel). 2020. PMID: 33561074 Free PMC article. Review.
Cited by
-
Role of sex and sex hormones in PD-L1 expression in NSCLC: clinical and therapeutic implications.Front Oncol. 2023 Oct 24;13:1210297. doi: 10.3389/fonc.2023.1210297. eCollection 2023. Front Oncol. 2023. PMID: 37941543 Free PMC article. Review.
-
The Efficacy of Ginsenoside Rg3 Combined with First-line Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer in China: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.Front Pharmacol. 2021 Mar 18;11:630825. doi: 10.3389/fphar.2020.630825. eCollection 2020. Front Pharmacol. 2021. PMID: 33815097 Free PMC article. Review.
-
Immune checkpoint inhibitors as first-line therapy for non-small cell lung cancer: A systematic evaluation and meta-analysis.Hum Vaccin Immunother. 2023 Dec 31;19(1):2169531. doi: 10.1080/21645515.2023.2169531. Epub 2023 Jan 30. Hum Vaccin Immunother. 2023. PMID: 36715018 Free PMC article.
-
An Indirect Comparison Between Nivolumab + Ipilimumab + Two Cycles of Chemotherapy vs. Pembrolizumab + Chemotherapy as First-Line Treatment for Metastatic Non-Small Cell Lung Cancer.Front Oncol. 2021 Sep 13;11:698199. doi: 10.3389/fonc.2021.698199. eCollection 2021. Front Oncol. 2021. PMID: 34589422 Free PMC article.
-
Comprehensive Transcriptomic Analysis Reveals the Role of the Immune Checkpoint HLA-G Molecule in Cancers.Front Immunol. 2021 Jul 1;12:614773. doi: 10.3389/fimmu.2021.614773. eCollection 2021. Front Immunol. 2021. PMID: 34276642 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

