In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2
- PMID: 32679055
- PMCID: PMC7361110
- DOI: 10.1016/j.antiviral.2020.104878
In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2
Abstract
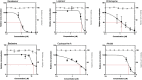
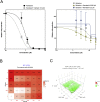
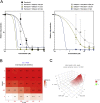
In response to the current pandemic caused by the novel SARS-CoV-2, identifying and validating effective therapeutic strategies is more than ever necessary. We evaluated the in vitro antiviral activities of a shortlist of compounds, known for their cellular broad-spectrum activities, together with drugs that are currently under evaluation in clinical trials for COVID-19 patients. We report the antiviral effect of remdesivir, lopinavir, chloroquine, umifenovir, berberine and cyclosporine A in Vero E6 cells model of SARS-CoV-2 infection, with estimated 50% inhibitory concentrations of 0.99, 5.2, 1.38, 3.5, 10.6 and 3 μM, respectively. Virus-directed plus host-directed drug combinations were also investigated. We report a strong antagonism between remdesivir and berberine, in contrast with remdesivir/diltiazem, for which we describe high levels of synergy, with mean Loewe synergy scores of 12 and peak values above 50. Combination of host-directed drugs with direct acting antivirals underscore further validation in more physiological models, yet they open up interesting avenues for the treatment of COVID-19.
Keywords: Antivirals; Berberine; COVID-19; Diltiazem; Drug combination; Remdesivir.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro.Antiviral Res. 2020 Jun;178:104786. doi: 10.1016/j.antiviral.2020.104786. Epub 2020 Apr 3. Antiviral Res. 2020. PMID: 32251767 Free PMC article.
-
Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2.Biomed Pharmacother. 2020 Nov;131:110668. doi: 10.1016/j.biopha.2020.110668. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861965 Free PMC article. Review.
-
Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro.Cell Res. 2020 Mar;30(3):269-271. doi: 10.1038/s41422-020-0282-0. Epub 2020 Feb 4. Cell Res. 2020. PMID: 32020029 Free PMC article. No abstract available.
-
Remdesivir against COVID-19 and Other Viral Diseases.Clin Microbiol Rev. 2020 Oct 14;34(1):e00162-20. doi: 10.1128/CMR.00162-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33055231 Free PMC article. Review.
-
SARS-CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and other Drugs for the Treatment of the New Coronavirus.Curr Med Chem. 2020;27(27):4536-4541. doi: 10.2174/0929867327666200416131117. Curr Med Chem. 2020. PMID: 32297571
Cited by
-
Antimicrobial activities of Diltiazem Hydrochloride: drug repurposing approach.PeerJ. 2024 Sep 23;12:e17809. doi: 10.7717/peerj.17809. eCollection 2024. PeerJ. 2024. PMID: 39329140 Free PMC article.
-
Fangchinoline induces antiviral response by suppressing STING degradation.J Pharm Anal. 2024 Jun;14(6):100972. doi: 10.1016/j.jpha.2024.100972. Epub 2024 Mar 28. J Pharm Anal. 2024. PMID: 39027910 Free PMC article.
-
QSPR/QSAR study of antiviral drugs modeled as multigraphs by using TI's and MLR method to treat COVID-19 disease.Sci Rep. 2024 Jun 7;14(1):13150. doi: 10.1038/s41598-024-63007-w. Sci Rep. 2024. PMID: 38849399
-
Management of immunosuppression in lung transplant recipients and COVID-19 outcomes: an observational retrospective cohort-study.BMC Infect Dis. 2024 May 28;24(1):536. doi: 10.1186/s12879-024-09269-1. BMC Infect Dis. 2024. PMID: 38807049 Free PMC article.
-
Bile acids and coronavirus disease 2019.Acta Pharm Sin B. 2024 May;14(5):1939-1950. doi: 10.1016/j.apsb.2024.02.011. Epub 2024 Feb 13. Acta Pharm Sin B. 2024. PMID: 38799626 Free PMC article. Review.
References
-
- Cao B., Wang Y., Wen D., Liu W., Wang Jingli, Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li Huadong, Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li Hui, Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang Juan, Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. - DOI - PMC - PubMed
-
- Chen C., Zhang Yi, Huang J., Yin P., Cheng Z., Wu J., Chen S., Zhang Yongxi, Chen B., Lu M., Luo Y., Ju L., Zhang J., Wang X. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020;2020 doi: 10.1101/2020.03.17.20037432. 03.17.20037432. - DOI
-
- Chen Z., Hu J., Zhang Zongwei, Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Zhan. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020;2020 doi: 10.1101/2020.03.22.20040758. 03.22.20040758. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

