Dexamethasone in Hospitalized Patients with Covid-19
- PMID: 32678530
- PMCID: PMC7383595
- DOI: 10.1056/NEJMoa2021436
Dexamethasone in Hospitalized Patients with Covid-19
Abstract
Background: Coronavirus disease 2019 (Covid-19) is associated with diffuse lung damage. Glucocorticoids may modulate inflammation-mediated lung injury and thereby reduce progression to respiratory failure and death.
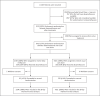
Methods: In this controlled, open-label trial comparing a range of possible treatments in patients who were hospitalized with Covid-19, we randomly assigned patients to receive oral or intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 days or to receive usual care alone. The primary outcome was 28-day mortality. Here, we report the final results of this assessment.
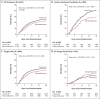
Results: A total of 2104 patients were assigned to receive dexamethasone and 4321 to receive usual care. Overall, 482 patients (22.9%) in the dexamethasone group and 1110 patients (25.7%) in the usual care group died within 28 days after randomization (age-adjusted rate ratio, 0.83; 95% confidence interval [CI], 0.75 to 0.93; P<0.001). The proportional and absolute between-group differences in mortality varied considerably according to the level of respiratory support that the patients were receiving at the time of randomization. In the dexamethasone group, the incidence of death was lower than that in the usual care group among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81) and among those receiving oxygen without invasive mechanical ventilation (23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94) but not among those who were receiving no respiratory support at randomization (17.8% vs. 14.0%; rate ratio, 1.19; 95% CI, 0.92 to 1.55).
Conclusions: In patients hospitalized with Covid-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support. (Funded by the Medical Research Council and National Institute for Health Research and others; RECOVERY ClinicalTrials.gov number, NCT04381936; ISRCTN number, 50189673.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
Research in the Context of a Pandemic.N Engl J Med. 2021 Feb 25;384(8):755-757. doi: 10.1056/NEJMe2024638. Epub 2020 Jul 17. N Engl J Med. 2021. PMID: 32678528 Free PMC article. No abstract available.
-
The RECOVERY Platform.N Engl J Med. 2021 Feb 25;384(8):757-758. doi: 10.1056/NEJMe2025674. Epub 2020 Jul 21. N Engl J Med. 2021. PMID: 32706531 Free PMC article. No abstract available.
-
Steroids in ARDS: more light is being shed.Intensive Care Med. 2020 Nov;46(11):2108-2110. doi: 10.1007/s00134-020-06230-z. Epub 2020 Sep 4. Intensive Care Med. 2020. PMID: 32886206 Free PMC article. No abstract available.
-
Steroids for sepsis and ARDS: this eternal controversy remains with COVID-19.Lancet. 2020 Oct 24;396(10259):e61-e62. doi: 10.1016/S0140-6736(20)32132-2. Epub 2020 Oct 9. Lancet. 2020. PMID: 33045189 Free PMC article. No abstract available.
-
Pragmatic trials with prespecified subgroups: what oncologists can learn from COVID-19.Nat Rev Clin Oncol. 2021 Jan;18(1):7-8. doi: 10.1038/s41571-020-00448-y. Nat Rev Clin Oncol. 2021. PMID: 33139895 Free PMC article.
-
COVID-19 and critical care capacity: Can we mitigate demand?Respirology. 2022 Feb;27(2):107-108. doi: 10.1111/resp.14193. Epub 2021 Dec 5. Respirology. 2022. PMID: 34865289 No abstract available.
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results.N Engl J Med. 2021 Feb 11;384(6):497-511. doi: 10.1056/NEJMoa2023184. Epub 2020 Dec 2. N Engl J Med. 2021. PMID: 33264556 Free PMC article. Clinical Trial.
-
Effect of dexamethasone in patients with ARDS and COVID-19 - prospective, multi-centre, open-label, parallel-group, randomised controlled trial (REMED trial): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Mar 1;22(1):172. doi: 10.1186/s13063-021-05116-9. Trials. 2021. PMID: 33648568 Free PMC article.
-
[Inhaled glucocorticoids in treatment of covid-19].Vnitr Lek. 2021 Winter;67(6):328-329. Vnitr Lek. 2021. PMID: 35459373 Review. Czech.
Cited by
-
Risk factors for in-hospital mortality in older patients with acute respiratory distress syndrome due to COVID-19: a retrospective cohort study.BMC Geriatr. 2024 Oct 26;24(1):878. doi: 10.1186/s12877-024-05411-5. BMC Geriatr. 2024. PMID: 39462358 Free PMC article.
-
Precision Medicine in Acute Respiratory Distress Syndrome: Progress, Challenges, and the Road ahead.Clin Chest Med. 2024 Dec;45(4):835-848. doi: 10.1016/j.ccm.2024.08.005. Epub 2024 Sep 20. Clin Chest Med. 2024. PMID: 39443001 Review.
-
Clustering based on renal and inflammatory admission parameters in critically ill patients admitted to the ICU.PLoS One. 2024 Nov 1;19(11):e0307938. doi: 10.1371/journal.pone.0307938. eCollection 2024. PLoS One. 2024. PMID: 39485788 Free PMC article.
-
One-year mortality and associated factors in older hospitalized COVID-19 survivors: a Nationwide Cohort Study in Korea.Sci Rep. 2024 Oct 22;14(1):24889. doi: 10.1038/s41598-024-76871-3. Sci Rep. 2024. PMID: 39438611 Free PMC article.
-
Extracorporeal Membrane Oxygenation instead of Invasive Mechanical Ventilation in a Patient with Severe COVID-19-associated Acute Respiratory Distress Syndrome.Am J Respir Crit Care Med. 2021 Jun 15;203(12):1571-1573. doi: 10.1164/rccm.202102-0259LE. Am J Respir Crit Care Med. 2021. PMID: 33901416 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- G0701652/MRC_/Medical Research Council/United Kingdom
- MC_PC_19056/MRC_/Medical Research Council/United Kingdom
- MC_UU_00017/3/MRC_/Medical Research Council/United Kingdom
- MC_U137686861/MRC_/Medical Research Council/United Kingdom
- MR/K025643/1/MRC_/Medical Research Council/United Kingdom
- RP-2014-05-019/DH_/Department of Health/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_UU_00002/14/MRC_/Medical Research Council/United Kingdom
- MR/S001751/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_20062/MRC_/Medical Research Council/United Kingdom
- MC_PC_20029/MRC_/Medical Research Council/United Kingdom
- MC_PC_18033/MRC_/Medical Research Council/United Kingdom
- 25350/CRUK_/Cancer Research UK/United Kingdom
- MC_U137686860/MRC_/Medical Research Council/United Kingdom
- MC_UU_12026/4/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
