Bliss' and Loewe's additive and synergistic effects in Plasmodium falciparum growth inhibition by AMA1-RON2L, RH5, RIPR and CyRPA antibody combinations
- PMID: 32678144
- PMCID: PMC7366652
- DOI: 10.1038/s41598-020-67877-8
Bliss' and Loewe's additive and synergistic effects in Plasmodium falciparum growth inhibition by AMA1-RON2L, RH5, RIPR and CyRPA antibody combinations
Abstract
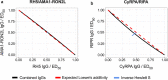
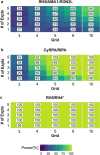
Plasmodium invasion of red blood cells involves malaria proteins, such as reticulocyte-binding protein homolog 5 (RH5), RH5 interacting protein (RIPR), cysteine-rich protective antigen (CyRPA), apical membrane antigen 1 (AMA1) and rhoptry neck protein 2 (RON2), all of which are blood-stage malaria vaccine candidates. So far, vaccines containing AMA1 alone have been unsuccessful in clinical trials. However, immunization with AMA1 bound with RON2L (AMA1-RON2L) induces better protection against P. falciparum malaria in Aotus monkeys. We therefore sought to determine whether combinations of RH5, RIPR, CyRPA and AMA1-RON2L antibodies improve their biological activities and sought to develop a robust method for determination of synergy or additivity in antibody combinations. Rabbit antibodies against AMA1-RON2L, RH5, RIPR or CyRPA were tested either alone or in combinations in P. falciparum growth inhibition assay to determine Bliss' and Loewe's additivities. The AMA1-RON2L/RH5 combination consistently demonstrated an additive effect while the CyRPA/RIPR combination showed a modest synergistic effect with Hewlett's [Formula: see text] Additionally, we provide a publicly-available, online tool to aid researchers in analyzing and planning their own synergy experiments. This study supports future blood-stage vaccine development by providing a solid methodology to evaluate additive and/or synergistic (or antagonistic) effect of vaccine-induced antibodies.
Conflict of interest statement
SJD is a named inventor on patent applications relating to RH5 and/or other malaria vaccines and immunization regimens. The other authors do not have conflict of interest.
Figures



Similar articles
-
Plasmodium falciparum Cysteine-Rich Protective Antigen (CyRPA) Elicits Detectable Levels of Invasion-Inhibitory Antibodies during Natural Infection in Humans.Infect Immun. 2022 Jan 25;90(1):e0037721. doi: 10.1128/IAI.00377-21. Epub 2021 Oct 25. Infect Immun. 2022. PMID: 34694918 Free PMC article.
-
RH5.1-CyRPA-Ripr antigen combination vaccine shows little improvement over RH5.1 in a preclinical setting.Front Cell Infect Microbiol. 2022 Dec 20;12:1049065. doi: 10.3389/fcimb.2022.1049065. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36605129 Free PMC article.
-
Development of an improved blood-stage malaria vaccine targeting the essential RH5-CyRPA-RIPR invasion complex.Nat Commun. 2024 Jun 7;15(1):4857. doi: 10.1038/s41467-024-48721-3. Nat Commun. 2024. PMID: 38849365 Free PMC article.
-
Identification and Immune Assessment of T Cell Epitopes in Five Plasmodium falciparum Blood Stage Antigens to Facilitate Vaccine Candidate Selection and Optimization.Front Immunol. 2021 Jul 7;12:690348. doi: 10.3389/fimmu.2021.690348. eCollection 2021. Front Immunol. 2021. PMID: 34305923 Free PMC article.
-
The RH5-CyRPA-Ripr Complex as a Malaria Vaccine Target.Trends Parasitol. 2020 Jun;36(6):545-559. doi: 10.1016/j.pt.2020.04.003. Epub 2020 Apr 28. Trends Parasitol. 2020. PMID: 32359873 Free PMC article. Review.
Cited by
-
The malaria blood stage antigen PfCyRPA formulated with the TLR-4 agonist adjuvant GLA-SE elicits parasite growth inhibitory antibodies in experimental animals.Malar J. 2023 Jul 15;22(1):210. doi: 10.1186/s12936-023-04638-8. Malar J. 2023. PMID: 37454145 Free PMC article.
-
Interactions of plumbagin with five common antibiotics against Staphylococcus aureus in vitro.PLoS One. 2024 Jan 26;19(1):e0297493. doi: 10.1371/journal.pone.0297493. eCollection 2024. PLoS One. 2024. PMID: 38277418 Free PMC article.
-
Strain-Dependent Inhibition of Erythrocyte Invasion by Monoclonal Antibodies Against Plasmodium falciparum CyRPA.Front Immunol. 2021 Aug 10;12:716305. doi: 10.3389/fimmu.2021.716305. eCollection 2021. Front Immunol. 2021. PMID: 34447381 Free PMC article.
-
Monoclonal antibodies for malaria prevention.Mol Ther. 2022 May 4;30(5):1810-1821. doi: 10.1016/j.ymthe.2022.04.001. Epub 2022 Apr 5. Mol Ther. 2022. PMID: 35395399 Free PMC article. Review.
-
Vaccine-induced human monoclonal antibodies to PfRH5 show broadly neutralizing activity against P. falciparum clinical isolates.NPJ Vaccines. 2024 Oct 24;9(1):198. doi: 10.1038/s41541-024-00986-x. NPJ Vaccines. 2024. PMID: 39448626 Free PMC article.
References
-
- World Health Organization. World Malaria Report 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/ (2018).
-
- Gaur, D., Chitnis, C. E. & Chauhan, V. S. Molecular basis of erythrocyte invasion by Plasmodium merozoites. In Advances in Malaria Research 33–86 (Wiley, New York, 2016). 10.1002/9781118493816.ch3
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

