Auxin mediates the touch-induced mechanical stimulation of adventitious root formation under windy conditions in Brachypodium distachyon
- PMID: 32678030
- PMCID: PMC7364541
- DOI: 10.1186/s12870-020-02544-8
Auxin mediates the touch-induced mechanical stimulation of adventitious root formation under windy conditions in Brachypodium distachyon
Abstract
Background: It is widely perceived that mechanical or thigmomorphogenic stimuli, such as rubbing and bending by passing animals, wind, raindrop, and flooding, broadly influence plant growth and developmental patterning. In particular, wind-driven mechanical stimulation is known to induce the incidence of radial expansion and shorter and stockier statue. Wind stimulation also affects the adaptive propagation of the root system in various plant species. However, it is unknown how plants sense and transmit the wind-derived mechanical signals to launch appropriate responses, leading to the wind-adaptive root growth.
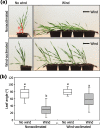
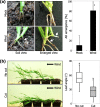
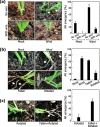
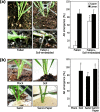
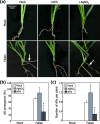
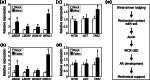
Results: Here, we found that Brachypodium distachyon, a model grass widely used for studies on bioenergy crops and cereals, efficiently adapts to wind-mediated lodging stress by forming adventitious roots (ARs) from nonroot tissues. Experimental dissection of wind stimuli revealed that not bending of the mesocotyls but physical contact of the leaf nodes with soil particles triggers the transcriptional induction of a group of potential auxin-responsive genes encoding WUSCHEL RELATED HOMEOBOX and LATERAL ORGAN BOUNDARIES DOMAIN transcription factors, which are likely to be involved in the induction of AR formation.
Conclusions: Our findings would contribute to further understanding molecular mechanisms governing the initiation and development of ARs, which will be applicable to crop agriculture in extreme wind climates.
Keywords: Adventitious root; Auxin; Brachypodium distachyon; Gravity; Lodging; Thigmomorphogenesis; Wind.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Genome-wide identification, molecular evolution, and expression analysis of auxin response factor (ARF) gene family in Brachypodium distachyon L.BMC Plant Biol. 2018 Dec 6;18(1):336. doi: 10.1186/s12870-018-1559-z. BMC Plant Biol. 2018. PMID: 30522432 Free PMC article.
-
Genome-Wide Transcript Profiling Reveals an Auxin-Responsive Transcription Factor, OsAP2/ERF-40, Promoting Rice Adventitious Root Development.Plant Cell Physiol. 2019 Oct 1;60(10):2343-2355. doi: 10.1093/pcp/pcz132. Plant Cell Physiol. 2019. PMID: 31318417
-
An atlas of Brachypodium distachyon lateral root development.Biol Open. 2024 Sep 15;13(9):bio060531. doi: 10.1242/bio.060531. Epub 2024 Sep 2. Biol Open. 2024. PMID: 39158386 Free PMC article.
-
Adventitious root formation in crops-Potato as an example.Physiol Plant. 2021 May;172(1):124-133. doi: 10.1111/ppl.13305. Epub 2020 Dec 21. Physiol Plant. 2021. PMID: 33305392 Review.
-
Initiation of vascular development.Physiol Plant. 2014 Jun;151(2):142-6. doi: 10.1111/ppl.12111. Epub 2013 Oct 21. Physiol Plant. 2014. PMID: 24111590 Review.
Cited by
-
Mechanically induced localisation of SECONDARY WALL INTERACTING bZIP is associated with thigmomorphogenic and secondary cell wall gene expression.Quant Plant Biol. 2024 May 3;5:e5. doi: 10.1017/qpb.2024.5. eCollection 2024. Quant Plant Biol. 2024. PMID: 38774130 Free PMC article.
-
Jasmonate inhibits adventitious root initiation through repression of CKX1 and activation of RAP2.6L transcription factor in Arabidopsis.J Exp Bot. 2021 Oct 26;72(20):7107-7118. doi: 10.1093/jxb/erab358. J Exp Bot. 2021. PMID: 34329421 Free PMC article.
-
Touch signaling and thigmomorphogenesis are regulated by complementary CAMTA3- and JA-dependent pathways.Sci Adv. 2022 May 20;8(20):eabm2091. doi: 10.1126/sciadv.abm2091. Epub 2022 May 20. Sci Adv. 2022. PMID: 35594358 Free PMC article.
-
Evaluating Root Mechanosensing Response in Rice.Methods Mol Biol. 2022;2494:25-35. doi: 10.1007/978-1-0716-2297-1_3. Methods Mol Biol. 2022. PMID: 35467198
-
Quantification of maize brace root formation after vertical stalk displacement.MicroPubl Biol. 2024 Apr 2;2024:10.17912/micropub.biology.001189. doi: 10.17912/micropub.biology.001189. eCollection 2024. MicroPubl Biol. 2024. PMID: 38633871 Free PMC article.
References
-
- Shah AN, Tanveer M, Rehman AU, Anjum SA, Iqbal J, Ahmad R. Lodging stress in cereal-effects and management: an overview. Environ Sci Pollut Res. 2017;24:5222–5237. - PubMed
-
- Takahashi H, Jaffe MJ. Thigmomorphogenesis: the relationship of mechanical perturbation to elicitor-like activity and ethylene production. Physiol Plant. 1984;61:405–411. - PubMed
-
- Chehab EW, Eich E, Braam J. Thigmomorphogenesis: a complex plant response to mechano-stimulation. J Exp Bot. 2009;60:43–56. - PubMed
-
- Mi C, Zhang X, Li S, Yang J, Zhu D, Yang Y. Assessment of environment lodging stress for maize using fuzzy synthetic evaluation. Math Comp Modeling. 2011;54:1053–1060.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

