Endothelial damage and dysfunction in acute graft-versus-host disease
- PMID: 32675225
- PMCID: PMC8327719
- DOI: 10.3324/haematol.2020.253716
Endothelial damage and dysfunction in acute graft-versus-host disease
Abstract
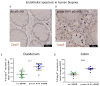
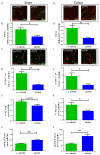
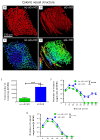
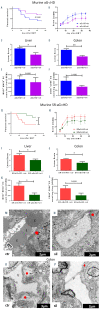
Clinical studies suggested that endothelial dysfunction and damage could be involved in the development and severity of acute graft-versus-host disease (aGVHD). Accordingly, we found increased percentage of apoptotic Casp3+ blood vessels in duodenal and colonic mucosa biopsies of patients with severe aGVHD. In murine experimental aGVHD, we detected severe microstructural endothelial damage and reduced endothelial pericyte coverage accompanied by reduced expression of endothelial tight junction proteins leading to increased endothelial leakage in aGVHD target organs. During intestinal aGVHD, colonic vasculature structurally changed, reflected by increased vessel branching and vessel diameter. Because recent data demonstrated an association of endothelium-related factors and steroid refractory aGVHD (SR-aGVHD), we analyzed human biopsies and murine tissues from SR-aGVHD. We found extensive tissue damage but low levels of alloreactive T cell infiltration in target organs, providing the rationale for T-cell independent SR-aGVHD treatment strategies. Consequently, we tested the endothelium-protective PDE5 inhibitor sildenafil, which reduced apoptosis and improved metabolic activity of endothelial cells in vitro. Accordingly, sildenafil treatment improved survival and reduced target organ damage during experimental SR-aGVHD. Our results demonstrate extensive damage, structural changes, and dysfunction of the vasculature during aGVHD. Therapeutic intervention by endothelium-protecting agents is an attractive approach for SR-aGVHD complementing current anti-inflammatory treatment options.
Figures



Similar articles
-
Differential protein expression in endothelial cells exposed to serum from patients with acute graft-vs-host disease, depending on steroid response.J Cell Mol Med. 2023 May;27(9):1227-1238. doi: 10.1111/jcmm.17712. Epub 2023 Apr 4. J Cell Mol Med. 2023. PMID: 37016544 Free PMC article.
-
Acute Graft-vs.-Host Disease-Associated Endothelial Activation in vitro Is Prevented by Defibrotide.Front Immunol. 2019 Oct 9;10:2339. doi: 10.3389/fimmu.2019.02339. eCollection 2019. Front Immunol. 2019. PMID: 31649666 Free PMC article. Clinical Trial.
-
Endothelial microparticles carrying hedgehog-interacting protein induce continuous endothelial damage in the pathogenesis of acute graft-versus-host disease.Am J Physiol Cell Physiol. 2016 May 15;310(10):C821-35. doi: 10.1152/ajpcell.00372.2015. Epub 2016 Mar 23. Am J Physiol Cell Physiol. 2016. PMID: 27009877
-
[Research Progress on Endothelial-Cell Injury in the Acute Graft-Versus-Host Disease].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016 Jun;24(3):954-7. doi: 10.7534/j.issn.1009-2137.2016.03.059. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016. PMID: 27342541 Review. Chinese.
-
Acute graft-versus-host disease-challenge for a broader application of allogeneic hematopoietic cell transplantation.Curr Stem Cell Res Ther. 2006 May;1(2):203-12. doi: 10.2174/157488806776956896. Curr Stem Cell Res Ther. 2006. PMID: 18220867 Review.
Cited by
-
Murine cytomegalovirus reactivation concomitant with acute graft-versus-host disease is controlled by antibodies.JCI Insight. 2023 Mar 8;8(5):e149648. doi: 10.1172/jci.insight.149648. JCI Insight. 2023. PMID: 36719764 Free PMC article.
-
Differential protein expression in endothelial cells exposed to serum from patients with acute graft-vs-host disease, depending on steroid response.J Cell Mol Med. 2023 May;27(9):1227-1238. doi: 10.1111/jcmm.17712. Epub 2023 Apr 4. J Cell Mol Med. 2023. PMID: 37016544 Free PMC article.
-
Recent advances in graft-versus-host disease.Fac Rev. 2023 Mar 6;12:4. doi: 10.12703/r/12-4. eCollection 2023. Fac Rev. 2023. PMID: 36923700 Free PMC article. Review.
-
The Clinical Value of Procalcitonin in the Neutropenic Period After Allogeneic Hematopoietic Stem Cell Transplantation.Front Immunol. 2022 Apr 25;13:843067. doi: 10.3389/fimmu.2022.843067. eCollection 2022. Front Immunol. 2022. PMID: 35547733 Free PMC article.
-
Phase II, Open-Label Clinical Trial of Urinary-Derived Human Chorionic Gonadotropin/Epidermal Growth Factor for Life-Threatening Acute Graft-versus-Host Disease.Transplant Cell Ther. 2023 Aug;29(8):509.e1-509.e8. doi: 10.1016/j.jtct.2023.05.021. Epub 2023 Jun 4. Transplant Cell Ther. 2023. PMID: 37279855 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

