Immobilization after injury alters extracellular matrix and stem cell fate
- PMID: 32673290
- PMCID: PMC7524473
- DOI: 10.1172/JCI136142
Immobilization after injury alters extracellular matrix and stem cell fate
Abstract
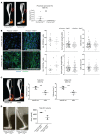
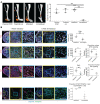
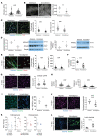
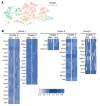
Cells sense the extracellular environment and mechanical stimuli and translate these signals into intracellular responses through mechanotransduction, which alters cell maintenance, proliferation, and differentiation. Here we use a mouse model of trauma-induced heterotopic ossification (HO) to examine how cell-extrinsic forces impact mesenchymal progenitor cell (MPC) fate. After injury, single-cell (sc) RNA sequencing of the injury site reveals an early increase in MPC genes associated with pathways of cell adhesion and ECM-receptor interactions, and MPC trajectories to cartilage and bone. Immunostaining uncovers active mechanotransduction after injury with increased focal adhesion kinase signaling and nuclear translocation of transcriptional coactivator TAZ, inhibition of which mitigates HO. Similarly, joint immobilization decreases mechanotransductive signaling, and completely inhibits HO. Joint immobilization decreases collagen alignment and increases adipogenesis. Further, scRNA sequencing of the HO site after injury with or without immobilization identifies gene signatures in mobile MPCs correlating with osteogenesis, and signatures from immobile MPCs with adipogenesis. scATAC-seq in these same MPCs confirm that in mobile MPCs, chromatin regions around osteogenic genes are open, whereas in immobile MPCs, regions around adipogenic genes are open. Together these data suggest that joint immobilization after injury results in decreased ECM alignment, altered MPC mechanotransduction, and changes in genomic architecture favoring adipogenesis over osteogenesis, resulting in decreased formation of HO.
Keywords: Bone Biology; Bone development; Cell migration/adhesion; Extracellular matrix; Stem cells.
Conflict of interest statement
Figures




Similar articles
-
The HIF-1α/PLOD2 axis integrates extracellular matrix organization and cell metabolism leading to aberrant musculoskeletal repair.Bone Res. 2024 Mar 12;12(1):17. doi: 10.1038/s41413-024-00320-0. Bone Res. 2024. PMID: 38472175 Free PMC article.
-
High Frequency Spectral Ultrasound Imaging Detects Early Heterotopic Ossification in Rodents.Stem Cells Dev. 2021 May 1;30(9):473-484. doi: 10.1089/scd.2021.0011. Epub 2021 Apr 19. Stem Cells Dev. 2021. PMID: 33715398 Free PMC article.
-
Wnt/ß-catenin-mediated p53 suppression is indispensable for osteogenesis of mesenchymal progenitor cells.Cell Death Dis. 2021 May 21;12(6):521. doi: 10.1038/s41419-021-03758-w. Cell Death Dis. 2021. PMID: 34021120 Free PMC article.
-
Mechanically induced osteogenic lineage commitment of stem cells.Stem Cell Res Ther. 2013;4(5):107. doi: 10.1186/scrt318. Stem Cell Res Ther. 2013. PMID: 24004875 Free PMC article. Review.
-
How is mechanobiology involved in mesenchymal stem cell differentiation toward the osteoblastic or adipogenic fate?J Cell Physiol. 2019 Aug;234(8):12133-12141. doi: 10.1002/jcp.28099. Epub 2019 Jan 11. J Cell Physiol. 2019. PMID: 30633367 Review.
Cited by
-
Single-Cell Analyses of Heterotopic Ossification: Characteristics of Injury-Related Senescent Fibroblasts.J Inflamm Res. 2022 Sep 24;15:5579-5593. doi: 10.2147/JIR.S369376. eCollection 2022. J Inflamm Res. 2022. PMID: 36185637 Free PMC article.
-
Influence of PHA Substrate Surface Characteristics on the Functional State of Endothelial Cells.J Funct Biomater. 2023 Feb 2;14(2):85. doi: 10.3390/jfb14020085. J Funct Biomater. 2023. PMID: 36826884 Free PMC article.
-
Contemporary perspectives on heterotopic ossification.JCI Insight. 2022 Jul 22;7(14):e158996. doi: 10.1172/jci.insight.158996. JCI Insight. 2022. PMID: 35866484 Free PMC article. Review.
-
Acetabular Reaming Is a Reliable Model to Produce and Characterize Periarticular Heterotopic Ossification of the Hip.Stem Cells Transl Med. 2022 Aug 23;11(8):876-888. doi: 10.1093/stcltm/szac042. Stem Cells Transl Med. 2022. PMID: 35758541 Free PMC article.
-
Key Genes Identified in Nonsyndromic Microtia by the Analysis of Transcriptomics and Proteomics.ACS Omega. 2022 May 13;7(20):16917-16927. doi: 10.1021/acsomega.1c07059. eCollection 2022 May 24. ACS Omega. 2022. PMID: 35647449 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

