Differential Wound Healing Capacity of Mesenchymal Stem Cell-Derived Exosomes Originated From Bone Marrow, Adipose Tissue and Umbilical Cord Under Serum- and Xeno-Free Condition
- PMID: 32671095
- PMCID: PMC7327117
- DOI: 10.3389/fmolb.2020.00119
Differential Wound Healing Capacity of Mesenchymal Stem Cell-Derived Exosomes Originated From Bone Marrow, Adipose Tissue and Umbilical Cord Under Serum- and Xeno-Free Condition
Abstract
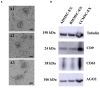
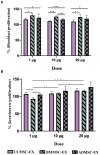
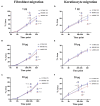
Exosomes are nano-scale and closed membrane vesicles which are promising for therapeutic applications due to exosome-enclosed therapeutic molecules such as DNA, small RNAs, proteins and lipids. Recently, it has been demonstrated that mesenchymal stem cell (MSC)-derived exosomes have capacity to regulate many biological events associated with wound healing process, such as cell proliferation, cell migration and blood vessel formation. This study investigated the regenerative potentials for cutaneous tissue, in regard to growth factors associated with wound healing and skin cell proliferation and migration, by exosomes released from primary MSCs originated from bone marrow (BM), adipose tissue (AD), and umbilical cord (UC) under serum- and xeno-free condition. We found crucial wound healing-mediated growth factors, such as vascular endothelial growth factor A (VEGF-A), fibroblast growth factor 2 (FGF-2), hepatocyte growth factor (HGF), and platelet-derived growth factor BB (PDGF-BB) in exosomes derived from all three MSC sources. However, expression levels of these growth factors in exosomes were influenced by MSC origins, especially transforming growth factor beta (TGF-β) was only detected in UCMSC-derived exosomes. All exosomes released by three MSCs sources induced keratinocyte and fibroblast proliferation and migration; and, the induction of cell migration is a dependent manner with the higher dose of exosomes was used (20 μg), the faster migration rate was observed. Additionally, the influences of exosomes on cell proliferation and migration was associated with exosome origins and also target cells of exosomes that the greatest induction of primary dermal fibroblasts belongs to BMMSC-derived exosomes and keratinocytes belongs to UCMSC-derived exosomes. Data from this study indicated that BMMSCs and UCMSCs under clinical condition secreted exosomes are promising to develop into therapeutic products for wound healing treatment.
Keywords: ADMSC-derived exosomes; BMMSC-derived exosomes; UCMSC-derived exosomes; exosomes; growth factors; mesenchymal stem cells; wound healing.
Copyright © 2020 Hoang, Nguyen, Nguyen, Nguyen, Do, Dang, Dam, Bui, Trinh, Vu, Hoang, Thanh and Than.
Figures



Similar articles
-
Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition.Stem Cell Res Ther. 2021 Aug 3;12(1):434. doi: 10.1186/s13287-021-02517-0. Stem Cell Res Ther. 2021. PMID: 34344478 Free PMC article.
-
Manufacturing exosomes for wound healing: Comparative analysis of culture media.PLoS One. 2024 Nov 14;19(11):e0313697. doi: 10.1371/journal.pone.0313697. eCollection 2024. PLoS One. 2024. PMID: 39541412 Free PMC article.
-
Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro.Stem Cells Dev. 2015 Jul 15;24(14):1635-47. doi: 10.1089/scd.2014.0316. Epub 2015 May 20. Stem Cells Dev. 2015. PMID: 25867197 Free PMC article.
-
Stem cell-derived exosomes: emerging therapeutic opportunities for wound healing.Stem Cell Res Ther. 2023 Apr 26;14(1):107. doi: 10.1186/s13287-023-03345-0. Stem Cell Res Ther. 2023. PMID: 37101197 Free PMC article. Review.
-
Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan.Regen Ther. 2022 Jun 15;21:73-80. doi: 10.1016/j.reth.2022.05.008. eCollection 2022 Dec. Regen Ther. 2022. PMID: 35785041 Free PMC article. Review.
Cited by
-
3D bioprinted mesenchymal stromal cells in skin wound repair.Front Surg. 2022 Oct 14;9:988843. doi: 10.3389/fsurg.2022.988843. eCollection 2022. Front Surg. 2022. PMID: 36311952 Free PMC article. Review.
-
Manufacture and Quality Control of Human Umbilical Cord-Derived Mesenchymal Stem Cell Sheets for Clinical Use.Cells. 2022 Sep 1;11(17):2732. doi: 10.3390/cells11172732. Cells. 2022. PMID: 36078137 Free PMC article.
-
Adipose stem cells-released extracellular vesicles as a next-generation cargo delivery vehicles: a survey of minimal information implementation, mass production and functional modification.Stem Cell Res Ther. 2022 May 3;13(1):182. doi: 10.1186/s13287-022-02849-5. Stem Cell Res Ther. 2022. PMID: 35505389 Free PMC article.
-
Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate.Int J Nanomedicine. 2020 Nov 24;15:9355-9371. doi: 10.2147/IJN.S281890. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33262592 Free PMC article. Review.
-
Research trends of mesenchymal stem cells application in orthopedics: A bibliometric analysis of the past 2 decades.Front Public Health. 2022 Sep 26;10:1021818. doi: 10.3389/fpubh.2022.1021818. eCollection 2022. Front Public Health. 2022. PMID: 36225768 Free PMC article.
References
-
- Ahn S. Y., Park W. S., Kim Y. E., Sung D. K., Sung S. I., Ahn J. Y., et al. (2018). Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 50:26 10.1038/s12276-018-0055-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

