Assessing Anti-HCMV Cell Mediated Immune Responses in Transplant Recipients and Healthy Controls Using a Novel Functional Assay
- PMID: 32670891
- PMCID: PMC7332694
- DOI: 10.3389/fcimb.2020.00275
Assessing Anti-HCMV Cell Mediated Immune Responses in Transplant Recipients and Healthy Controls Using a Novel Functional Assay
Abstract
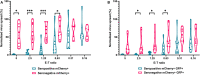
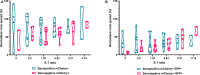
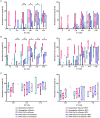
HCMV infection, reinfection or reactivation occurs in 60% of untreated solid organ transplant (SOT) recipients. Current clinical approaches to HCMV management include pre-emptive and prophylactic antiviral treatment strategies. The introduction of immune monitoring to better stratify patients at risk of viraemia and HCMV mediated disease could improve clinical management. Current approaches quantify T cell IFNγ responses specific for predominantly IE and pp65 proteins ex vivo, as a proxy for functional control of HCMV in vivo. However, these approaches have only a limited predictive ability. We measured the IFNγ T cell responses to an expanded panel of overlapping peptide pools specific for immunodominant HCMV proteins IE1/2, pp65, pp71, gB, UL144, and US3 in a cohort of D+R- kidney transplant recipients in a longitudinal analysis. Even with this increased antigen diversity, the results show that while all patients had detectable T cell responses, this did not correlate with control of HCMV replication in some. We wished to develop an assay that could directly measure anti-HCMV cell-mediated immunity. We evaluated three approaches, stimulation of PBMC with (i) whole HCMV lysate or (ii) a defined panel of immunodominant HCMV peptides, or (iii) fully autologous infected cells co-cultured with PBMC or isolated CD8+ T cells or NK cells. Stimulation with HCMV lysate often generated non-specific antiviral responses while stimulation with immunodominant HCMV peptide pools produced responses which were not necessarily antiviral despite strong IFNγ production. We demonstrated that IFNγ was only a minor component of secreted antiviral activity. Finally, we used an antiviral assay system to measure the effect of whole PBMC, and isolated CD8+ T cells and NK cells to control HCMV in infected autologous dermal fibroblasts. The results show that both PBMC and especially CD8+ T cells from HCMV seropositive donors have highly specific antiviral activity against HCMV. In addition, we were able to show that NK cells were also antiviral, but the level of this control was highly variable between donors and not dependant on HCMV seropositivity. Using this approach, we show that non-viraemic D+R+ SOT recipients had significant and specific antiviral activity against HCMV.
Keywords: T cells; antiviral; cell-mediated immunity; cytomegalovirus; herpesvirus; host-pathogen interactions; secreted immunity; transplantation.
Copyright © 2020 Houldcroft, Jackson, Lim, Sedikides, Davies, Atkinson, McIntosh, Remmerswaal, Okecha, Bemelman, Stanton, Reeves and Wills.
Figures

Similar articles
-
Normalizing ELISPOT responses to T-cell counts: a novel approach for quantification of HCMV-specific CD4(+) and CD8(+) T-cell responses in kidney transplant recipients.J Clin Virol. 2014 Sep;61(1):65-73. doi: 10.1016/j.jcv.2014.05.017. Epub 2014 Jun 4. J Clin Virol. 2014. PMID: 24961915
-
ELISPOT assays with pp65 peptides or whole HCMV antigen are reliable predictors of immune control of HCMV infection in seropositive kidney transplant recipients.J Med Virol. 2023 Feb;95(2):e28507. doi: 10.1002/jmv.28507. J Med Virol. 2023. PMID: 36655741 Free PMC article.
-
Immune Control of Human Cytomegalovirus (HCMV) Infection in HCMV-Seropositive Solid Organ Transplant Recipients: The Predictive Role of Different Immunological Assays.Cells. 2024 Aug 8;13(16):1325. doi: 10.3390/cells13161325. Cells. 2024. PMID: 39195215 Free PMC article.
-
The CD4+ T Cell Response to Human Cytomegalovirus in Healthy and Immunocompromised People.Front Cell Infect Microbiol. 2020 May 19;10:202. doi: 10.3389/fcimb.2020.00202. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32509591 Free PMC article. Review.
-
The next generation recombinant human cytomegalovirus vaccine candidates-beyond gB.Vaccine. 2012 Nov 19;30(49):6980-90. doi: 10.1016/j.vaccine.2012.09.056. Epub 2012 Oct 3. Vaccine. 2012. PMID: 23041121 Review.
Cited by
-
HCMV carriage in the elderly diminishes anti-viral functionality of the adaptive immune response resulting in virus replication at peripheral sites.Front Immunol. 2022 Dec 15;13:1083230. doi: 10.3389/fimmu.2022.1083230. eCollection 2022. Front Immunol. 2022. PMID: 36591233 Free PMC article.
-
A Novel Multiplexed Enzyme-Linked Immunosorbent Assay for the Detection of IgG Seroreactivity to Cytomegalovirus (CMV) UL144.J Clin Microbiol. 2021 Jul 19;59(8):e0096421. doi: 10.1128/JCM.00964-21. Epub 2021 Jul 19. J Clin Microbiol. 2021. PMID: 34076473 Free PMC article.
-
IL-10-Secreting CD8+ T Cells Specific for Human Cytomegalovirus (HCMV): Generation, Maintenance and Phenotype.Pathogens. 2022 Dec 13;11(12):1530. doi: 10.3390/pathogens11121530. Pathogens. 2022. PMID: 36558866 Free PMC article.
-
Monoclonal antibodies targeting nonstructural viral antigens can activate ADCC against human cytomegalovirus.J Clin Invest. 2021 Feb 15;131(4):e139296. doi: 10.1172/JCI139296. J Clin Invest. 2021. PMID: 33586678 Free PMC article.
-
Identifying high-confidence variants in human cytomegalovirus genomes sequenced from clinical samples.Virus Evol. 2022 Dec 5;8(2):veac114. doi: 10.1093/ve/veac114. eCollection 2022. Virus Evol. 2022. PMID: 37091479 Free PMC article.
References
-
- Abate D., Saldan A., Fiscon M., Cofano S., Paciolla A., Furian L., et al. (2010). Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J. Infect. Dis. 202, 585–594. 10.1086/654931 - DOI - PubMed
-
- Baraniak I., Kropff B., Ambrose L., McIntosh M., McLean G. R., Pichon S., et al. . (2018). Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc. Natl. Acad. Sci. U.S.A. 115, 6273–6278. 10.1073/pnas.1800224115 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

