Administration of Metabiotics Extracted From Probiotic Lactobacillus rhamnosus MD 14 Inhibit Experimental Colorectal Carcinogenesis by Targeting Wnt/β-Catenin Pathway
- PMID: 32670864
- PMCID: PMC7326139
- DOI: 10.3389/fonc.2020.00746
Administration of Metabiotics Extracted From Probiotic Lactobacillus rhamnosus MD 14 Inhibit Experimental Colorectal Carcinogenesis by Targeting Wnt/β-Catenin Pathway
Abstract
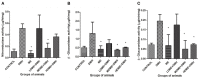
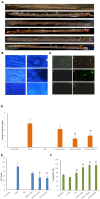
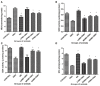
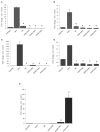
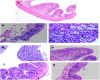
Background and Objective: The cellular microenvironment, diet, and lifestyle play a key role in the occurrence of colorectal cancer. Due to its rising trend, attempts are being made to devise novel biointerventions as adjunct to conventional therapies to prevent this deadly disease. "Metabiotics," the beneficial metabolic signatures of probiotics are emerging as potential anticancer agent due to their ability to alter metabolic processes in the gut lumen and reduce the severity of colon carcinogenesis. Although beneficial attributes of metabiotics have been elucidated in vitro, yet their anticancer mechanism in vivo needs to be explored. Thus, the present study was performed to envisage anticancer potential of metabiotic extract obtained from indigenous probiotic, Lactobacillus rhamnosus MD 14, in early experimental colon carcinogenesis. Materials and Methods: Sprague-Dawley rats were daily administered with low, medium, and high dose of metabiotic extract orally along with a single dose of weekly intraperitoneal injection of 1,2-dimethylhydrazine up to 6 weeks and monitored for the markers of early colon carcinogenesis. Results: It was observed that the medium dose of metabiotic extract attenuated early colon carcinogenesis by reducing fecal procarcinogenic enzymes, oxidants, aberrant crypt foci, vis-à-vis downregulating oncogenes [K-ras, β-catenin, Cox-2, nuclear factor kappa B (NF-κB)] and upregulating tumor suppressor p53 gene leading to almost normal colon histology. Conclusions: It can be suggested that metabiotics modulate experimental colorectal cancer and could be used as a promising alternative of probiotics, particularly in immunocompromised individuals.
Keywords: aberrant crypt foci; bioactive substances; colon cancer; metabiotics; probiotics.
Copyright © 2020 Sharma and Shukla.
Figures

Similar articles
-
A Narrative Review on the Advance of Probiotics to Metabiotics.J Microbiol Biotechnol. 2024 Mar 28;34(3):487-494. doi: 10.4014/jmb.2311.11023. Epub 2024 Jan 29. J Microbiol Biotechnol. 2024. PMID: 38247208 Free PMC article. Review.
-
Probiotics (Lactobacillus acidophilus and Lactobacillus rhamnosus GG) in Conjunction with Celecoxib (selective COX-2 inhibitor) Modulated DMH-Induced Early Experimental Colon Carcinogenesis.Nutr Cancer. 2018 Aug-Sep;70(6):946-955. doi: 10.1080/01635581.2018.1490783. Epub 2018 Sep 5. Nutr Cancer. 2018. PMID: 30183370
-
Probiotics Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in Sprague Dawley rats.Nutr Cancer. 2013;65(1):84-91. doi: 10.1080/01635581.2013.741746. Nutr Cancer. 2013. PMID: 23368917
-
Antigenotoxicity and Cytotoxic Potentials of Metabiotics Extracted from Isolated Probiotic, Lactobacillus rhamnosus MD 14 on Caco-2 and HT-29 Human Colon Cancer Cells.Nutr Cancer. 2020;72(1):110-119. doi: 10.1080/01635581.2019.1615514. Epub 2019 Jul 3. Nutr Cancer. 2020. PMID: 31266374
-
Metabiotics: One Step ahead of Probiotics; an Insight into Mechanisms Involved in Anticancerous Effect in Colorectal Cancer.Front Microbiol. 2016 Dec 2;7:1940. doi: 10.3389/fmicb.2016.01940. eCollection 2016. Front Microbiol. 2016. PMID: 27994577 Free PMC article. Review.
Cited by
-
Research Trend and Detailed Insights into the Molecular Mechanisms of Food Bioactive Compounds against Cancer: A Comprehensive Review with Special Emphasis on Probiotics.Cancers (Basel). 2022 Nov 8;14(22):5482. doi: 10.3390/cancers14225482. Cancers (Basel). 2022. PMID: 36428575 Free PMC article. Review.
-
A Narrative Review on the Advance of Probiotics to Metabiotics.J Microbiol Biotechnol. 2024 Mar 28;34(3):487-494. doi: 10.4014/jmb.2311.11023. Epub 2024 Jan 29. J Microbiol Biotechnol. 2024. PMID: 38247208 Free PMC article. Review.
-
Probiotics and postbiotics in colorectal cancer: Prevention and complementary therapy.World J Gastroenterol. 2022 Jul 21;28(27):3370-3382. doi: 10.3748/wjg.v28.i27.3370. World J Gastroenterol. 2022. PMID: 36158273 Free PMC article. Review.
-
Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain-Gut-Microbiome Axis.Biomolecules. 2021 Jul 7;11(7):1000. doi: 10.3390/biom11071000. Biomolecules. 2021. PMID: 34356624 Free PMC article. Review.
-
Role of Gut Microbiota and Probiotics in Colorectal Cancer: Onset and Progression.Microorganisms. 2021 May 10;9(5):1021. doi: 10.3390/microorganisms9051021. Microorganisms. 2021. PMID: 34068653 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

