Cough as an adverse effect on inhalation pharmaceutical products
- PMID: 32668011
- PMCID: PMC7443471
- DOI: 10.1111/bph.15197
Cough as an adverse effect on inhalation pharmaceutical products
Abstract
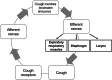
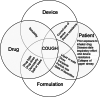
Cough is an adverse effect that may hinder the delivery of drugs into the lungs. Chemical or mechanical stimulants activate the transient receptor potential in some airway afferent nerves (C-fibres or A-fibres) to trigger cough. Types of inhaler device and drug, dose, excipients and formulation characteristics, including pH, tonicity, aerosol output and particle size may trigger cough by stimulating the cough receptors. Release of inflammatory mediators may increase the sensitivity of the cough receptors to stimulants. The cough-provoking effect of aerosols is enhanced by bronchoconstriction in diseased airways and reduces drug deposition in the target pulmonary regions. In this article, we review the factors by which inhalation products may cause cough.
Keywords: adverse effect; aerosol; cough; inhalation; pharmaceutical products.
© 2020 The British Pharmacological Society.
Conflict of interest statement
J.D.B. receives a 10% portion of royalties for the sale of Aridol™/Osmohaler™ that are paid to his prior employer, Royal Prince Alfred Hospital. He holds a minimum number of shares in the manufacturer Pharmaxis Ltd. In the past, he has acted as a consultant to Pharmaxis Ltd and the North American distributor of Aridol, Methapharm Pty Ltd.
Figures
Similar articles
-
Nebulized Mannitol, Particle Distribution, and Cough in Idiopathic Pulmonary Fibrosis.Respir Care. 2018 Nov;63(11):1407-1412. doi: 10.4187/respcare.06153. Epub 2018 Aug 28. Respir Care. 2018. PMID: 30154129
-
Regional sensitivity of human airways to capsaicin-induced cough.Am Rev Respir Dis. 1992 May;145(5):1191-5. doi: 10.1164/ajrccm/145.5.1191. Am Rev Respir Dis. 1992. PMID: 1586064 Clinical Trial.
-
Computationally efficient analysis of particle transport and deposition in a human whole-lung-airway model. Part II: Dry powder inhaler application.Comput Biol Med. 2017 May 1;84:247-253. doi: 10.1016/j.compbiomed.2016.10.025. Epub 2016 Nov 3. Comput Biol Med. 2017. PMID: 27836120
-
Post-inhalation cough with therapeutic aerosols: Formulation considerations.Adv Drug Deliv Rev. 2020;165-166:127-141. doi: 10.1016/j.addr.2020.05.003. Epub 2020 May 14. Adv Drug Deliv Rev. 2020. PMID: 32417367 Review.
-
Air and soul: the science and application of aerosol therapy.Respir Care. 2010 Jul;55(7):911-21. Respir Care. 2010. PMID: 20587104 Review.
Cited by
-
Impact of nebulization versus metered-dose inhaler utilization on viral particle dispersion in patients with COVID-19.J Infect Prev. 2024 Jul 22:17571774241266420. doi: 10.1177/17571774241266420. Online ahead of print. J Infect Prev. 2024. PMID: 39544634 Free PMC article.
-
Comparative Analysis of Inhaled Insulin With Other Types in Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis.Cureus. 2022 Apr 1;14(4):e23731. doi: 10.7759/cureus.23731. eCollection 2022 Apr. Cureus. 2022. PMID: 35509734 Free PMC article. Review.
-
Deep Inspiration-Provoked Cough: A Sign of Cough Reflex Arc Hypersensitivity.Lung. 2021 Oct;199(5):501-505. doi: 10.1007/s00408-021-00476-0. Epub 2021 Sep 15. Lung. 2021. PMID: 34528127
-
Nebulised Isotonic Hydroxychloroquine Aerosols for Potential Treatment of COVID-19.Pharmaceutics. 2021 Aug 14;13(8):1260. doi: 10.3390/pharmaceutics13081260. Pharmaceutics. 2021. PMID: 34452220 Free PMC article.
-
Enhanced curcumin loaded nanocellulose: a possible inhalable nanotherapeutic to treat COVID-19.Cellulose (Lond). 2022;29(3):1821-1840. doi: 10.1007/s10570-021-04391-8. Epub 2022 Jan 4. Cellulose (Lond). 2022. PMID: 35002106 Free PMC article.
References
-
- Abdulqawi, R. , Dockry, R. , Holt, K. , Layton, G. , McCarthy, B. G. , Ford, A. P. , & Smith, J. A. (2015). P2X3 receptor antagonist (AF‐219) in refractory chronic cough: A randomised, double‐blind, placebo‐controlled phase 2 study. Lancet, 385, 1,198–1,205. - PubMed
-
- Adcock, J. J. (2009). TRPV1 receptors in sensitisation of cough and pain reflexes. Pulmonary Pharmacology & Therapeutics, 22, 65–70. - PubMed
-
- Anderson, S. D. , Brannan, J. , Spring, J. , Spalding, N. , Rodwell, L. T. , Chan, K. I. M. , … Clark, A. R. (1997). A new method for bronchial‐provocation testing in asthmatic subjects using a dry powder of mannitol. American Journal of Respiratory and Critical Care Medicine, 156, 758–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical