Effect of Gemcitabine based chemotherapy on the immunogenicity of pancreatic tumour cells and T-cells
- PMID: 32661823
- PMCID: PMC7820186
- DOI: 10.1007/s12094-020-02429-0
Effect of Gemcitabine based chemotherapy on the immunogenicity of pancreatic tumour cells and T-cells
Abstract
Purpose: Chemotherapy for advanced pancreatic cancer has limited efficacy due to the difficultly of treating established tumours and the evolution of tumour resistance. Chemotherapies for pancreatic cancer are typically studied for their cytotoxic properties rather than for their ability to increase the immunogenicity of pancreatic tumour cells. In this study Gemcitabine in combination with immune modulatory chemotherapies Oxaliplatin, zoledronic acid and pomalidomide was studied to determine how combination therapy alters the immunogenicity of pancreatic tumour cell lines and subsequent T-cell responses.
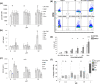
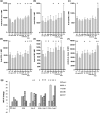
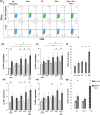
Methods: Pancreatic tumour cell lines were stimulated with the chemotherapeutic agents and markers of immune recognition were assessed. The effect of chemotherapeutic agents on DC function was measured using uptake of CFSE-stained PANC-1 cells, changes in markers of maturation and their ability to activate CD8+ T-cells. The effect of chemotherapeutic agents on T-cell priming prior to activation using anti-CD3 and anti-CD28 antibodies was determined by measuring IFN-γ expression and Annexin V staining using flow cytometry.
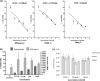
Results: These agents demonstrate both additive and inhibitory properties on a range of markers of immunogenicity. Gemcitabine was notable for its ability to induce the upregulation of human leukocyte antigen and checkpoints on pancreatic tumour cell lines whilst inhibiting T-cell activation. Pomalidomide demonstrated immune modulatory properties on dendritic cells and T-cells, even in the presence of gemcitabine.
Discussion: These data highlight the complex interactions of different agents in the modulation of tumour immunogenicity and immune cell activation and emphasise the complexity in rationally designing chemo immunogenic combinations for use with immunotherapy.
Keywords: Chemotherapy; Gemcitabine; Immunotherapy; Pancreatic cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Concomitant gemcitabine therapy negatively affects DC vaccine-induced CD8(+) T-cell and B-cell responses but improves clinical efficacy in a murine pancreatic carcinoma model.Cancer Immunol Immunother. 2014 Apr;63(4):321-33. doi: 10.1007/s00262-013-1510-y. Epub 2014 Jan 3. Cancer Immunol Immunother. 2014. PMID: 24384835 Free PMC article.
-
Gemcitabine-treated pancreatic cancer cell medium induces the specific CTL antitumor activity by stimulating the maturation of dendritic cells.Int Immunopharmacol. 2014 Mar;19(1):10-6. doi: 10.1016/j.intimp.2013.12.022. Epub 2014 Jan 3. Int Immunopharmacol. 2014. PMID: 24389382
-
Proapoptotic and antiapoptotic proteins of the Bcl-2 family regulate sensitivity of pancreatic cancer cells toward gemcitabine and T-cell-mediated cytotoxicity.J Immunother. 2015 Apr;38(3):116-26. doi: 10.1097/CJI.0000000000000073. J Immunother. 2015. PMID: 25751501
-
Recent updates on the role of chemotherapy in pancreatic cancer.Semin Oncol. 2005 Aug;32(4 Suppl 6):S1-3. doi: 10.1053/j.seminoncol.2005.06.022. Semin Oncol. 2005. PMID: 16143160 Review.
-
Microorganisms in chemotherapy for pancreatic cancer: An overview of current research and future directions.Int J Biol Sci. 2021 Jun 26;17(10):2666-2682. doi: 10.7150/ijbs.59117. eCollection 2021. Int J Biol Sci. 2021. PMID: 34326701 Free PMC article. Review.
Cited by
-
Advances in immunotherapy for biliary tract cancers.Chin Med J (Engl). 2024 Mar 5;137(5):524-532. doi: 10.1097/CM9.0000000000002759. Epub 2023 Aug 29. Chin Med J (Engl). 2024. PMID: 37646139 Free PMC article. Review.
-
Modulation of Type I Interferon Responses to Influence Tumor-Immune Cross Talk in PDAC.Front Cell Dev Biol. 2022 Feb 22;10:816517. doi: 10.3389/fcell.2022.816517. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35273962 Free PMC article. Review.
-
Immunogenic Cell Death: An Emerging Target in Gastrointestinal Cancers.Cells. 2022 Sep 28;11(19):3033. doi: 10.3390/cells11193033. Cells. 2022. PMID: 36230995 Free PMC article. Review.
-
Translational Learnings in the Development of Chemo-Immunotherapy Combination to Bypass the Cold Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma.Front Oncol. 2022 May 18;12:835502. doi: 10.3389/fonc.2022.835502. eCollection 2022. Front Oncol. 2022. PMID: 35664786 Free PMC article. Review.
-
The immune modifying effects of chemotherapy and advances in chemo-immunotherapy.Pharmacol Ther. 2022 Aug;236:108111. doi: 10.1016/j.pharmthera.2022.108111. Epub 2022 Jan 10. Pharmacol Ther. 2022. PMID: 35016920 Free PMC article. Review.
References
-
- Smyth EN, Bapat B, Ball DE, André T, Kaye JA. Metastatic pancreatic adenocarcinoma treatment patterns, health care resource use, and outcomes in France and the United Kingdom between 2009 and 2012: a retrospective study. Clin Ther. 2015;37:1301–1316. doi: 10.1016/j.clinthera.2015.03.016. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

