Sex- and Region-Specific Differences in the Transcriptomes of Rat Microglia from the Brainstem and Cervical Spinal Cord
- PMID: 32661056
- PMCID: PMC7569313
- DOI: 10.1124/jpet.120.266171
Sex- and Region-Specific Differences in the Transcriptomes of Rat Microglia from the Brainstem and Cervical Spinal Cord
Abstract
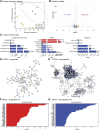
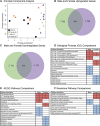
The neural control system underlying breathing is sexually dimorphic with males being more vulnerable to dysfunction. Microglia also display sex differences, and their role in the architecture of brainstem respiratory rhythm circuitry and modulation of cervical spinal cord respiratory plasticity is becoming better appreciated. To further understand the molecular underpinnings of these sex differences, we performed RNA sequencing of immunomagnetically isolated microglia from brainstem and cervical spinal cord of adult male and female rats. We used various bioinformatics tools (Gene Ontology, Kyoto Encyclopedia of Genes and Genomes, Reactome, STRING, MAGICTRICKS) to functionally categorize identified gene sets, as well as to pinpoint common transcriptional gene drivers that may be responsible for the observed transcriptomic differences. We found few sex differences in the microglial transcriptomes derived from the brainstem, but several hundred genes were differentially expressed by sex in cervical spinal microglia. Comparing brainstem and spinal microglia within and between sexes, we found that the major factor guiding transcriptomic differences was central nervous system (CNS) location rather than sex. We further identified key transcriptional drivers that may be responsible for the transcriptomic differences observed between sexes and CNS regions; enhancer of zeste homolog 2 emerged as the predominant driver of the differentially downregulated genes. We suggest that functional gene alterations identified in metabolism, transcription, and intercellular communication underlie critical microglial heterogeneity and sex differences in CNS regions that contribute to respiratory disorders categorized by dysfunction in neural control. These data will also serve as an important resource data base to advance our understanding of innate immune cell contributions to sex differences and the field of respiratory neural control. SIGNIFICANCE STATEMENT: The contributions of central nervous system (CNS) innate immune cells to sexually dimorphic differences in the neural circuitry controlling breathing are poorly understood. We identify key transcriptomic differences, and their transcriptional drivers, in microglia derived from the brainstem and the C3-C6 cervical spinal cord of healthy adult male and female rats. Gene alterations identified in metabolism, gene transcription, and intercellular communication likely underlie critical microglial heterogeneity and sex differences in these key CNS regions that contribute to the neural control of breathing.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Priming of Adult Incision Response by Early-Life Injury: Neonatal Microglial Inhibition Has Persistent But Sexually Dimorphic Effects in Adult Rats.J Neurosci. 2019 Apr 17;39(16):3081-3093. doi: 10.1523/JNEUROSCI.1786-18.2019. Epub 2019 Feb 22. J Neurosci. 2019. PMID: 30796159 Free PMC article.
-
Modulation of microglial activation states by spinal cord stimulation in an animal model of neuropathic pain: Comparing high rate, low rate, and differential target multiplexed programming.Mol Pain. 2021 Jan-Dec;17:1744806921999013. doi: 10.1177/1744806921999013. Mol Pain. 2021. PMID: 33626981 Free PMC article.
-
Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats.Pain. 2018 Sep;159(9):1752-1763. doi: 10.1097/j.pain.0000000000001265. Pain. 2018. PMID: 29927790
-
Transcriptional profiling of microglia; current state of the art and future perspectives.Glia. 2020 Apr;68(4):740-755. doi: 10.1002/glia.23767. Epub 2019 Dec 17. Glia. 2020. PMID: 31846124 Free PMC article. Review.
-
Microglia Responses in Acute and Chronic Neurological Diseases: What Microglia-Specific Transcriptomic Studies Taught (and did Not Teach) Us.Front Aging Neurosci. 2017 Jul 21;9:227. doi: 10.3389/fnagi.2017.00227. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28785215 Free PMC article. Review.
Cited by
-
Severe Acute Respiratory Syndrome Coronavirus 2 Impact on the Central Nervous System: Are Astrocytes and Microglia Main Players or Merely Bystanders?ASN Neuro. 2020 Jan-Dec;12:1759091420954960. doi: 10.1177/1759091420954960. ASN Neuro. 2020. PMID: 32878468 Free PMC article. Review.
-
Microglial reactivity in brainstem chemosensory nuclei in response to hypercapnia.Front Physiol. 2024 Feb 27;15:1332355. doi: 10.3389/fphys.2024.1332355. eCollection 2024. Front Physiol. 2024. PMID: 38476146 Free PMC article.
-
Brain region- and sex-specific transcriptional profiles of microglia.Front Psychiatry. 2022 Aug 24;13:945548. doi: 10.3389/fpsyt.2022.945548. eCollection 2022. Front Psychiatry. 2022. PMID: 36090351 Free PMC article.
-
Role of microglia in blood pressure and respiratory responses to acute hypoxic exposure in rats.J Physiol Sci. 2022 Oct 13;72(1):26. doi: 10.1186/s12576-022-00848-y. J Physiol Sci. 2022. PMID: 36229778 Free PMC article.
-
The prenatal challenge with lipopolysaccharide and polyinosinic:polycytidylic acid disrupts CX3CL1-CX3CR1 and CD200-CD200R signalling in the brains of male rat offspring: a link to schizophrenia-like behaviours.J Neuroinflammation. 2020 Aug 23;17(1):247. doi: 10.1186/s12974-020-01923-0. J Neuroinflammation. 2020. PMID: 32829711 Free PMC article.
References
-
- Ahuja D, Mateika JH, Diamond MP, Badr MS. (2007) Ventilatory sensitivity to carbon dioxide before and after episodic hypoxia in women treated with testosterone. J Appl Physiol (1985) 102:1832–1838. - PubMed
-
- Baldy C, Fournier S, Boisjoly-Villeneuve S, Tremblay ME, Kinkead R. (2018) The influence of sex and neonatal stress on medullary microglia in rat pups. Exp Physiol 103:1192–1199. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
